
Why do HCl, \[\text{HN}{{\text{O}}_{\text{3}}}\]etc., show acidic character in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
Answer
603.3k+ views
Hint: Solve this question by applying the basics of properties and strength of acids. An acid is a substance or compound which can donate a proton. Strength of an acid depends on the ability of the compound to donate, when a compound can donate a proton easily, it is a strong acid.
Complete step by step answer:
An aqueous solution means a solution in which the solvent is water. Water is a polar compound which can dissociate as a proton and hydroxyl ion, i.e. –
\[{{\text{H}}_{\text{2}}}\text{O}\rightleftharpoons {{\text{H}}^{\text{+}}}\text{+O}{{\text{H}}^{\text{-}}}\]
Hydrochloric acid - HCl and nitric acid -\[\text{HN}{{\text{O}}_{\text{3}}}\] are strong acids in nature. It is because they dissociate completely as ions in aqueous solution. The reaction can be presented as –
\[\text{HCl}\to \text{ }{{\text{H}}^{\text{+}}}\text{+ C}{{\text{l}}^{\text{-}}}\]
\[\text{HN}{{\text{O}}_{\text{3}}}\to \text{ }{{\text{H}}^{\text{+}}}\text{+ NO}_{3}^{-}\]
Both hydrochloric and nitric acid increase the number of protons in the solution.
\[{{\text{H}}^{\text{+}}}\text{+ }{{\text{H}}_{\text{2}}}\text{O}\to {{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}}\]
Therefore, we can say that it shows acidic character in aqueous solutions.
On the other hand, compounds like glucose \[\text{(}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}})\] and alcohol (ROH) do not ionize or dissociate in water to give proton \[\text{(}{{\text{H}}^{\text{+}}})\]. Hydrogen does not separate as an individual ion. Therefore, it does not show acidic character.
Additional Information:
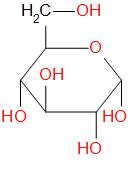
Glucose is a carbohydrate and a component of sucrose. It exists as a six membered ring. It is also known by names such as blood sugar, corn sugar, grape sugar, etc. The structure of D-glucose is given below –
 Note: Dissociation constant of strong acids is equal to one, it is because it dissociates completely, as we can see in the case of hydrochloric acid and nitric acid. Whereas, dissociation constant of strong acids is less than one, it is because weak acids do not dissociate completely. All organic acids are weak acids.
Note: Dissociation constant of strong acids is equal to one, it is because it dissociates completely, as we can see in the case of hydrochloric acid and nitric acid. Whereas, dissociation constant of strong acids is less than one, it is because weak acids do not dissociate completely. All organic acids are weak acids.
Complete step by step answer:
An aqueous solution means a solution in which the solvent is water. Water is a polar compound which can dissociate as a proton and hydroxyl ion, i.e. –
\[{{\text{H}}_{\text{2}}}\text{O}\rightleftharpoons {{\text{H}}^{\text{+}}}\text{+O}{{\text{H}}^{\text{-}}}\]
Hydrochloric acid - HCl and nitric acid -\[\text{HN}{{\text{O}}_{\text{3}}}\] are strong acids in nature. It is because they dissociate completely as ions in aqueous solution. The reaction can be presented as –
\[\text{HCl}\to \text{ }{{\text{H}}^{\text{+}}}\text{+ C}{{\text{l}}^{\text{-}}}\]
\[\text{HN}{{\text{O}}_{\text{3}}}\to \text{ }{{\text{H}}^{\text{+}}}\text{+ NO}_{3}^{-}\]
Both hydrochloric and nitric acid increase the number of protons in the solution.
\[{{\text{H}}^{\text{+}}}\text{+ }{{\text{H}}_{\text{2}}}\text{O}\to {{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}}\]
Therefore, we can say that it shows acidic character in aqueous solutions.
On the other hand, compounds like glucose \[\text{(}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}})\] and alcohol (ROH) do not ionize or dissociate in water to give proton \[\text{(}{{\text{H}}^{\text{+}}})\]. Hydrogen does not separate as an individual ion. Therefore, it does not show acidic character.
Additional Information:
Glucose is a carbohydrate and a component of sucrose. It exists as a six membered ring. It is also known by names such as blood sugar, corn sugar, grape sugar, etc. The structure of D-glucose is given below –

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




