
H-bonding is not present in:
(A) Glycerine
(B) Water
(C) Hydrogen sulphide
(D) Hydrogen fluoride
Answer
532.2k+ views
Hint: The hydrogen bonding is the electrostatic attraction present between the polar groups; when Hydrogen (H) is bonded with highly electronegative atom such as Nitrogen (N), Oxygen (O) or Fluorine (F) experience this kind of attraction.
Complete answer:
Let us analyse the given compounds;
Glycerine-
The chemical formula of glycerine is ${{C}_{3}}{{H}_{8}}{{O}_{3}}$ having the structure as described below-
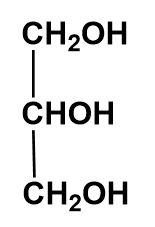
Here, we can see that the H is bonded to the highly electronegative atom i.e. oxygen. Thus, H – bonding is present in the glycerine.
Water-
The chemical formula of water is ${{H}_{2}}O$ having the structure as described below;
\[H-O-H\]
Here, we can see that the H is bonded to the highly electronegative atom i.e. oxygen. Thus, H – bonding is present in the water.
Hydrogen sulphide-
The chemical formula of hydrogen sulphide is ${{H}_{2}}S$ having structure as described below;
\[H-S-H\]
Here, we can see that the H is not bonded to any electronegative atom as of N, O and F. Thus, H – bonding is absent in hydrogen sulphide.
Hydrogen fluoride-
The chemical formula of hydrogen fluoride is HF having structure as described below;
\[H-F\]
Here, we can see that the H is bonded to the highly electronegative atom i.e. fluorine. Thus, H – bonding is present in the hydrogen fluoride.
Therefore, option (C) is correct.
Note:
We need to find the composition of the compounds to analyse the presence of H – bonding in those compounds. Water and Hydrogen fluoride clearly indicates the presence of electronegative elements i.e. oxygen and fluorine; forming hydrogen bonds.
Complete answer:
Let us analyse the given compounds;
Glycerine-
The chemical formula of glycerine is ${{C}_{3}}{{H}_{8}}{{O}_{3}}$ having the structure as described below-

Here, we can see that the H is bonded to the highly electronegative atom i.e. oxygen. Thus, H – bonding is present in the glycerine.
Water-
The chemical formula of water is ${{H}_{2}}O$ having the structure as described below;
\[H-O-H\]
Here, we can see that the H is bonded to the highly electronegative atom i.e. oxygen. Thus, H – bonding is present in the water.
Hydrogen sulphide-
The chemical formula of hydrogen sulphide is ${{H}_{2}}S$ having structure as described below;
\[H-S-H\]
Here, we can see that the H is not bonded to any electronegative atom as of N, O and F. Thus, H – bonding is absent in hydrogen sulphide.
Hydrogen fluoride-
The chemical formula of hydrogen fluoride is HF having structure as described below;
\[H-F\]
Here, we can see that the H is bonded to the highly electronegative atom i.e. fluorine. Thus, H – bonding is present in the hydrogen fluoride.
Therefore, option (C) is correct.
Note:
We need to find the composition of the compounds to analyse the presence of H – bonding in those compounds. Water and Hydrogen fluoride clearly indicates the presence of electronegative elements i.e. oxygen and fluorine; forming hydrogen bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




