
Have you ever noticed that when you reach into a bag of M and \[{M^1}\]s, that you are most likely to pull out a brown one?
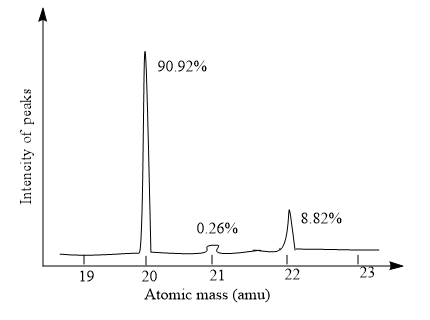
A group of students studied the mass spectrum of a new element they wanted to name cadmium is shown below.
What is the relative atomic mass of cadmium?

Answer
508.5k+ views
Hint: Here, given a graphical representation of the mass spectrum of the element candium with, the intensity of peaks on the y-axis and atomic mass is along the x-axis. And it shows three peaks. And the tallest peak will indicate the base peak and it is a particularly stable ion. And the mass spectrum, which represents a plot of intensity vs m/z ratio. And m/z ratio indicates the mass to charge ratio.
Complete answer:
In the given plot, the base peak shows at the atomic mass $20$ and the intensity of the peaks is equal to \[90.92\% \]. And the element with atomic mass $21$ shows the intensity of the peak at\[0.26\% \]. The element with atomic mass $22$ shows the peak with intensity \[8.82\% \].
The relative atomic mass of an element is the sum of the product of the percentage of peaks and the atomic mass and the formula of relative atomic mass can be written as,
\[\Sigma \% \,of\,peaks\, \times atomic\,mass\]
\[20 \to 90.92\% \]
\[21 \to 0.26\% \]
\[22 \to 8.82\% \]
Substitute the values in above equation, will get,
Relative atomic mass\[ = \dfrac{{90.92}}{{100}} \times 20\, + \,\dfrac{{0.26}}{{100}} \times 21\, + \,\dfrac{{8.82}}{{100}} \times 22\]
\[ = 18.184 + 0.0546 + 1.9404\]
\[ = 20.179\]
Hence, the relative atomic mass of candium is equal to \[20.179\].
Note:
From the given plot of the mass spectrum, we get the relative atomic mass of cadmium is equal to \[20.179\]. And it is a pattern that represents the distribution of the ions by using the mass to charge ratio. To read a mass spectrum, first, we have to identify the molecular ion and next to identify the fragmentation. And calculate the \[\Delta m\] of each peak. If there are any heteroatoms, identify them also, after that name the molecule.
Complete answer:
In the given plot, the base peak shows at the atomic mass $20$ and the intensity of the peaks is equal to \[90.92\% \]. And the element with atomic mass $21$ shows the intensity of the peak at\[0.26\% \]. The element with atomic mass $22$ shows the peak with intensity \[8.82\% \].
The relative atomic mass of an element is the sum of the product of the percentage of peaks and the atomic mass and the formula of relative atomic mass can be written as,
\[\Sigma \% \,of\,peaks\, \times atomic\,mass\]
\[20 \to 90.92\% \]
\[21 \to 0.26\% \]
\[22 \to 8.82\% \]
Substitute the values in above equation, will get,
Relative atomic mass\[ = \dfrac{{90.92}}{{100}} \times 20\, + \,\dfrac{{0.26}}{{100}} \times 21\, + \,\dfrac{{8.82}}{{100}} \times 22\]
\[ = 18.184 + 0.0546 + 1.9404\]
\[ = 20.179\]
Hence, the relative atomic mass of candium is equal to \[20.179\].
Note:
From the given plot of the mass spectrum, we get the relative atomic mass of cadmium is equal to \[20.179\]. And it is a pattern that represents the distribution of the ions by using the mass to charge ratio. To read a mass spectrum, first, we have to identify the molecular ion and next to identify the fragmentation. And calculate the \[\Delta m\] of each peak. If there are any heteroatoms, identify them also, after that name the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




