
What happens on thermal decomposition of $\text{ Ba}{{\left( {{\text{N}}_{\text{3}}} \right)}_{2}}\text{ }$? (Write the only equation of the reaction).
Answer
581.4k+ views
Hint: Thermal decomposition is a decomposition reaction of the substance in presence of heat. Alkaline earth metals form azide. This has general formula $\text{ M}{{\left( {{\text{N}}_{\text{3}}} \right)}_{2}}\text{ }$ . Alkaline earth azide undergoes thermal decomposition to give metal and nitrogen gas.
Complete Solution :
- The reaction of decomposition of substances caused by the heat is called thermal decomposition. The temperature at which the substance decomposed chemically is a decomposition temperature. This is an endothermic reaction and the heat is absorbed to break the chemical bonds of the compound.
Alkaline earth azide readily decomposes to give the alkaline earth metal and nitrogen on heating. Barium is an alkaline earth metal, It forms an azide.
- Barium azide is an inorganic compound. It has a formula as $\text{ Ba(}{{\text{N}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ }$ .it is explosive in nature.in barium azide structure one barium metal atom forms an ionic bond with the negatively charged nitrogen atom of triazide group $\text{ }\left( \overset{-}{\mathop{\text{N}}}\,\text{ = }\overset{+}{\mathop{\text{N}}}\,\text{ = }\overset{-}{\mathop{\text{N}}}\, \right)\text{ }$ . The general structure of barium azide is as follows:
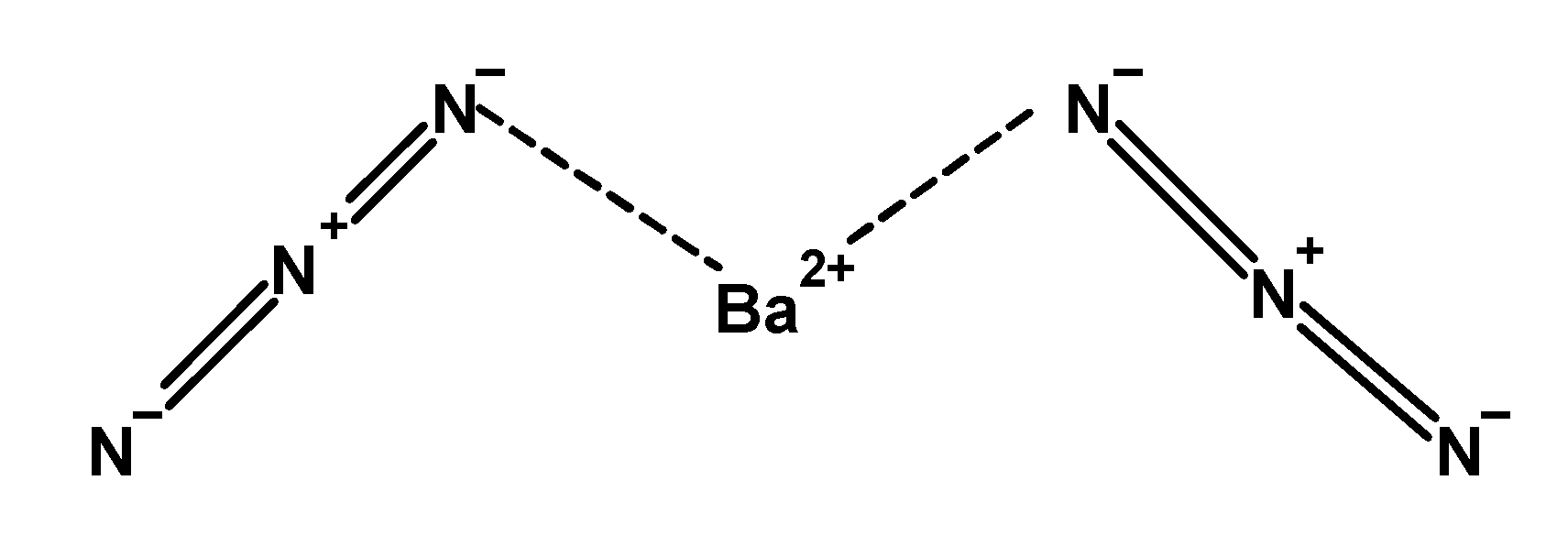
- Barium azide is used to prepare magnesium, sodium, lithium, rubidium, and zinc azides. On heating, at high temperature the barium azide undergoes thermal decomposition. The thermal decomposition of barium azide produces elemental barium with nitrogen gas. The thermal decomposition reaction is as shown below:
$\text{ Ba(}{{\text{N}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ }\xrightarrow{\Delta }\text{Ba + 3}{{\text{N}}_{\text{2 }}}\text{ }$
Thus, the thermal decomposition of barium azide produces barium metal and nitrogen gas.
Note: Note that, not every azide decomposes and liberates nitrogen on heating, some may remain in the solid-state as nitride. On heating of barium azide, barium is formed first then barium reacts with the barium azide and gives nitride. This is another reaction of thermal decomposition of barium azide. The reaction is as follows:
$\text{ Ba + Ba(}{{\text{N}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ }\xrightarrow{\Delta }\text{ B}{{\text{a}}_{\text{2}}}{{\text{N}}_{\text{2}}}\text{ + 2}{{\text{N}}_{\text{2}}}\text{ }$
Where $\text{ B}{{\text{a}}_{\text{2}}}{{\text{N}}_{\text{2}}}\text{ }$ is barium nitride.
Complete Solution :
- The reaction of decomposition of substances caused by the heat is called thermal decomposition. The temperature at which the substance decomposed chemically is a decomposition temperature. This is an endothermic reaction and the heat is absorbed to break the chemical bonds of the compound.
Alkaline earth azide readily decomposes to give the alkaline earth metal and nitrogen on heating. Barium is an alkaline earth metal, It forms an azide.
- Barium azide is an inorganic compound. It has a formula as $\text{ Ba(}{{\text{N}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ }$ .it is explosive in nature.in barium azide structure one barium metal atom forms an ionic bond with the negatively charged nitrogen atom of triazide group $\text{ }\left( \overset{-}{\mathop{\text{N}}}\,\text{ = }\overset{+}{\mathop{\text{N}}}\,\text{ = }\overset{-}{\mathop{\text{N}}}\, \right)\text{ }$ . The general structure of barium azide is as follows:

- Barium azide is used to prepare magnesium, sodium, lithium, rubidium, and zinc azides. On heating, at high temperature the barium azide undergoes thermal decomposition. The thermal decomposition of barium azide produces elemental barium with nitrogen gas. The thermal decomposition reaction is as shown below:
$\text{ Ba(}{{\text{N}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ }\xrightarrow{\Delta }\text{Ba + 3}{{\text{N}}_{\text{2 }}}\text{ }$
Thus, the thermal decomposition of barium azide produces barium metal and nitrogen gas.
Note: Note that, not every azide decomposes and liberates nitrogen on heating, some may remain in the solid-state as nitride. On heating of barium azide, barium is formed first then barium reacts with the barium azide and gives nitride. This is another reaction of thermal decomposition of barium azide. The reaction is as follows:
$\text{ Ba + Ba(}{{\text{N}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ }\xrightarrow{\Delta }\text{ B}{{\text{a}}_{\text{2}}}{{\text{N}}_{\text{2}}}\text{ + 2}{{\text{N}}_{\text{2}}}\text{ }$
Where $\text{ B}{{\text{a}}_{\text{2}}}{{\text{N}}_{\text{2}}}\text{ }$ is barium nitride.
Recently Updated Pages
Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Computer Science: Engaging Questions & Answers for Success

Class 10 Question and Answer - Your Ultimate Solutions Guide

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Trending doubts
A boat goes 24 km upstream and 28 km downstream in class 10 maths CBSE

State and explain Ohms law class 10 physics CBSE

Distinguish between soap and detergent class 10 chemistry CBSE

a Why did Mendel choose pea plants for his experiments class 10 biology CBSE

What is a "free hit" awarded for in limited-overs cricket?

Draw the diagram of the sectional view of the human class 10 biology CBSE




