
${H_3}PO_4^{}$ and ${H_2}S{O_4}$ are known as polybasic acids.
Reason: They have two or more than two protons per molecule of the acids.
A) Both Assertion and Reason are correct and Reason is the correct explanation of the Assertion.
B) Both Assertion and Reason are correct and Reason is not the correct explanation of the Assertion.
C) Assertion is correct but Reason is incorrect.
D) Both Assertion and Reason are incorrect.
Answer
581.1k+ views
Hint: Basicity of acids is defined as the number of hydrogen ions which can be produced by one molecule of the acid. If we know the structure of acid it will be easier to calculate the basicity of any acid. Phosphorus is in +5 oxidation state in phosphoric acid and sulphur is in +6 oxidation state in sulphuric acid.
Complete step by step answer:
To solve this type of question it is necessary that we should have the knowledge of drawing structures.
First let us calculate the oxidation number of the central metal atom in ${H_3}PO_4^{}$ and ${H_2}S{O_4}$.
Oxidation state in ${H_3}PO_4^{}$, $3 + x - 8 = 0$, x = 5 and In \[{H_2}S{O_4}\],$2 + x - 8 = 0$, x = 6
Also phosphorus have 5 electrons and sulphur have 6 electrons in their outermost shells.
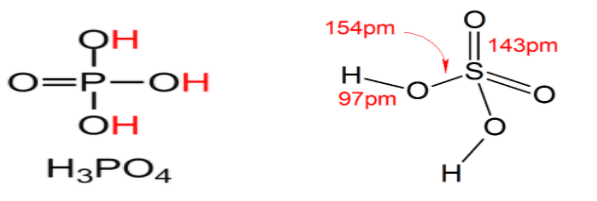
So we have structures, now let us talk about basicity.
Basicity is defined as the number of hydrogen ions produced by one molecule or number of –OH groups attached to that species. In these two figures we can easily see that there are 3 –OH groups in phosphoric acid and 2 –OH groups in sulphuric acid.
Hence Basicity of ${H_3}PO_4^{}$ is 3 and ${H_2}S{O_4}$ is 2 and polybasic acids are those which have basicity equal to two or greater than two.
Therefore, they both are polybasic acids . Our Assertion is correct.
And both acids are producing two protons or hydrogen ions so Our Reason in also correct.
So the correct option is: A.
Note: Actually sometimes students see the hydrogen directly attached to the central metal atom and give the answer of basicity which is wrong. We have to see the hydrogen which is attached to the oxygen atom. Always try to draw structure to give a final answer.
Complete step by step answer:
To solve this type of question it is necessary that we should have the knowledge of drawing structures.
First let us calculate the oxidation number of the central metal atom in ${H_3}PO_4^{}$ and ${H_2}S{O_4}$.
Oxidation state in ${H_3}PO_4^{}$, $3 + x - 8 = 0$, x = 5 and In \[{H_2}S{O_4}\],$2 + x - 8 = 0$, x = 6
Also phosphorus have 5 electrons and sulphur have 6 electrons in their outermost shells.

So we have structures, now let us talk about basicity.
Basicity is defined as the number of hydrogen ions produced by one molecule or number of –OH groups attached to that species. In these two figures we can easily see that there are 3 –OH groups in phosphoric acid and 2 –OH groups in sulphuric acid.
Hence Basicity of ${H_3}PO_4^{}$ is 3 and ${H_2}S{O_4}$ is 2 and polybasic acids are those which have basicity equal to two or greater than two.
Therefore, they both are polybasic acids . Our Assertion is correct.
And both acids are producing two protons or hydrogen ions so Our Reason in also correct.
So the correct option is: A.
Note: Actually sometimes students see the hydrogen directly attached to the central metal atom and give the answer of basicity which is wrong. We have to see the hydrogen which is attached to the oxygen atom. Always try to draw structure to give a final answer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




