
What is \[[{{H}^{+}}]\] in \[mol/L\] of a solution that is 0.20 M in $C{{H}_{3}}COONa$ and 0.10 M in $C{{H}_{3}}COOH$?
${{K}_{a}}$ for $C{{H}_{3}}COONa$ = $1.8\text{ x 1}{{\text{0}}^{-5}}$
(A) $3.5\text{ x 1}{{\text{0}}^{-4}}$
(B) $\text{1}\text{.1 x 1}{{\text{0}}^{-5}}$
(C) $\text{1}\text{.8 x 1}{{\text{0}}^{-5}}$
(D) $\text{9}\text{.0 x 1}{{\text{0}}^{-6}}$
Answer
584.7k+ views
Hint: It is important to know that acetic acid or rather ethanoic acid is a weak organic acid. In addition to that the conjugate base of acetic acid. This reduces the dissociation of the acid in accordance to Le chatlier's principle. Consider the effect of the common added to the solution while determining the dissociation constant.
Complete step-by-step answer:
Organic acids are in general considered to be weak acids due to relatively less stable conjugate base in comparison to a mineral acid.
The structure of ethanoic acid is :
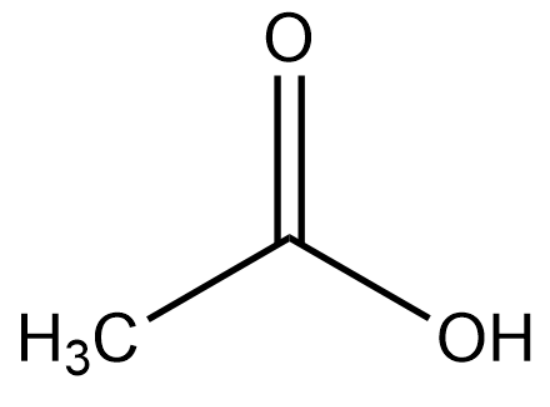
Upon deprotonation, the conjugate base of ethanoic acid becomes:
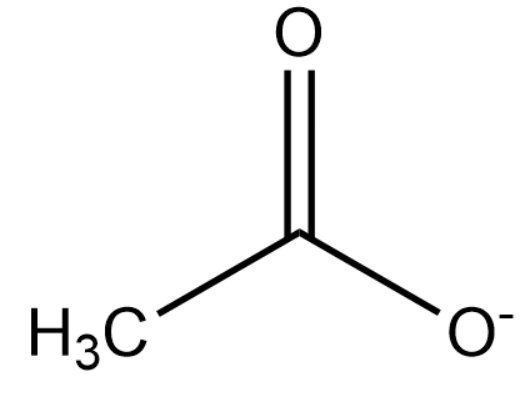 The ionisation of $C{{H}_{3}}COONa$ and $C{{H}_{3}}COOH$ is as follows:
The ionisation of $C{{H}_{3}}COONa$ and $C{{H}_{3}}COOH$ is as follows:
$\text{C}{{\text{H}}_{\text{3}}}\text{COONa }\to \text{ C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}\text{ + N}{{\text{a}}^{\text{+}}}$
$\text{C}{{\text{H}}_{\text{3}}}\text{COOH }\to \text{ C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}\text{ + }{{\text{H}}^{\text{+}}}$
Due to the common ion $\text{C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}$, the equilibrium for the dissociation of acid will shift backwards in accordance to Le Chatlier's principle.
We also know that,
${{\text{K}}_{\text{a}}}\text{ = }\dfrac{\text{ }\!\![\!\!\text{ C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}\text{ }\!\!]\!\!\text{ }\!\![\!\!\text{ }{{\text{H}}^{\text{+}}}\text{ }\!\!]\!\!\text{ }}{\text{C}{{\text{H}}_{\text{3}}}\text{COOH}}$
Here we will take the concentration as follows:
$\text{ }\!\![\!\!\text{ C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}]$ = 0.2 M
$\text{ }\!\![\!\!\text{ C}{{\text{H}}_{\text{3}}}\text{COOH}]$ = 0.1 M
\[{{\text{K}}_{\text{a}}}\]= $1.8\text{ x 1}{{\text{0}}^{-5}}$
Substituting the above values in the equation we get,
$1.8\text{ x 1}{{\text{0}}^{-5}}\text{ = }\dfrac{[0.2][{{\text{H}}^{+}}]}{[0.1]}$
$[{{\text{H}}^{+}}]\text{ = 9}\text{.0 x 1}{{\text{0}}^{-6}}\text{ mol }{{\text{L}}^{-1}}$
Therefore, the correct answer is option (D).
Additional information: In chemistry, organic compounds are chemical compounds containing carbon-hydrogen bonds in simple terms. Along with carbon and hydrogen, organic compounds contain atoms like oxygen, nitrogen and halogens( Cl, Br ,I, F).
The study of properties, reactions and their mechanisms and their synthesis is known as organic chemistry. Till now we have found and synthesised millions of organic compounds due to the catenation property of carbon.
Note: The common ion effect describes the effect of adding a common ion on the equilibrium of the new solution. The common ion effect generally decreases solubility of a solute. The equilibrium shifts to the left to relieve off the excess product formed. When a strong electrolyte is added to a solution of weak electrolyte, the solubility of weak electrolyte decreases leading to the formation of precipitate at the bottom of the testing apparatus.
Complete step-by-step answer:
Organic acids are in general considered to be weak acids due to relatively less stable conjugate base in comparison to a mineral acid.
The structure of ethanoic acid is :

Upon deprotonation, the conjugate base of ethanoic acid becomes:

$\text{C}{{\text{H}}_{\text{3}}}\text{COONa }\to \text{ C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}\text{ + N}{{\text{a}}^{\text{+}}}$
$\text{C}{{\text{H}}_{\text{3}}}\text{COOH }\to \text{ C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}\text{ + }{{\text{H}}^{\text{+}}}$
Due to the common ion $\text{C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}$, the equilibrium for the dissociation of acid will shift backwards in accordance to Le Chatlier's principle.
We also know that,
${{\text{K}}_{\text{a}}}\text{ = }\dfrac{\text{ }\!\![\!\!\text{ C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}\text{ }\!\!]\!\!\text{ }\!\![\!\!\text{ }{{\text{H}}^{\text{+}}}\text{ }\!\!]\!\!\text{ }}{\text{C}{{\text{H}}_{\text{3}}}\text{COOH}}$
Here we will take the concentration as follows:
$\text{ }\!\![\!\!\text{ C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}]$ = 0.2 M
$\text{ }\!\![\!\!\text{ C}{{\text{H}}_{\text{3}}}\text{COOH}]$ = 0.1 M
\[{{\text{K}}_{\text{a}}}\]= $1.8\text{ x 1}{{\text{0}}^{-5}}$
Substituting the above values in the equation we get,
$1.8\text{ x 1}{{\text{0}}^{-5}}\text{ = }\dfrac{[0.2][{{\text{H}}^{+}}]}{[0.1]}$
$[{{\text{H}}^{+}}]\text{ = 9}\text{.0 x 1}{{\text{0}}^{-6}}\text{ mol }{{\text{L}}^{-1}}$
Therefore, the correct answer is option (D).
Additional information: In chemistry, organic compounds are chemical compounds containing carbon-hydrogen bonds in simple terms. Along with carbon and hydrogen, organic compounds contain atoms like oxygen, nitrogen and halogens( Cl, Br ,I, F).
The study of properties, reactions and their mechanisms and their synthesis is known as organic chemistry. Till now we have found and synthesised millions of organic compounds due to the catenation property of carbon.
Note: The common ion effect describes the effect of adding a common ion on the equilibrium of the new solution. The common ion effect generally decreases solubility of a solute. The equilibrium shifts to the left to relieve off the excess product formed. When a strong electrolyte is added to a solution of weak electrolyte, the solubility of weak electrolyte decreases leading to the formation of precipitate at the bottom of the testing apparatus.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




