
How many grams of trinitrotoluene [${{C}_{7}}{{H}_{5}}{{(N{{O}_{2}})}_{3}}$] are in 0.00892 moles of TNT?
Answer
535.8k+ views
Hint: The formula that can be used for solving this question will be:
$Moles=\dfrac{Given\text{ }mass}{Molecular\,mass}$
To find the molecular mass you can use, the atomic mass of carbon as 12, the atomic mass of hydrogen as 1, the atomic mass of nitrogen as 14, and the atomic mass of oxygen as 16.
Complete answer:
TNT is an organic compound whose formula is given as [${{C}_{7}}{{H}_{5}}{{(N{{O}_{2}})}_{3}}$], in this compound there is an aromatic ring or benzene ring, at the first carbon there is a methyl group, and at 2, 4, 6 positions there is a nitro group. The structure of TNT is given below:
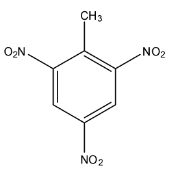
So, the given number of moles of trinitrotoluene is 0.00892 and we have to find the grams in these given moles. The formula that can be used for solving this question will be:
$Moles=\dfrac{Given\text{ }mass}{Molecular\,mass}$
So, we have to calculate the molecular mass. We can calculate the molecular mass by adding the atomic masses of the elements in the compound. The atomic mass of carbon is 12, the atomic mass of hydrogen is 1, the atomic mass of nitrogen is 14 and the atomic mass of oxygen is 16. The molecular mass will be:
$12\text{ x 7 + 1 x 5 + 3 x 14 + 6 x 16 = 227}$
So, now we can calculate the grams by multiplying the given moles with molecular mass.
Mass = moles x molecular mass
Mass = 0.00892 x 227 = 2.024 grams
So, there are 2.024 grams present in 0.00892 moles of TNT.
Note: We can also take the exact atomic mass of the atoms to solve the molecular mass of the compound. The mass is taken in grams because the atomic and molecular masses are in gram /mol.
$Moles=\dfrac{Given\text{ }mass}{Molecular\,mass}$
To find the molecular mass you can use, the atomic mass of carbon as 12, the atomic mass of hydrogen as 1, the atomic mass of nitrogen as 14, and the atomic mass of oxygen as 16.
Complete answer:
TNT is an organic compound whose formula is given as [${{C}_{7}}{{H}_{5}}{{(N{{O}_{2}})}_{3}}$], in this compound there is an aromatic ring or benzene ring, at the first carbon there is a methyl group, and at 2, 4, 6 positions there is a nitro group. The structure of TNT is given below:

So, the given number of moles of trinitrotoluene is 0.00892 and we have to find the grams in these given moles. The formula that can be used for solving this question will be:
$Moles=\dfrac{Given\text{ }mass}{Molecular\,mass}$
So, we have to calculate the molecular mass. We can calculate the molecular mass by adding the atomic masses of the elements in the compound. The atomic mass of carbon is 12, the atomic mass of hydrogen is 1, the atomic mass of nitrogen is 14 and the atomic mass of oxygen is 16. The molecular mass will be:
$12\text{ x 7 + 1 x 5 + 3 x 14 + 6 x 16 = 227}$
So, now we can calculate the grams by multiplying the given moles with molecular mass.
Mass = moles x molecular mass
Mass = 0.00892 x 227 = 2.024 grams
So, there are 2.024 grams present in 0.00892 moles of TNT.
Note: We can also take the exact atomic mass of the atoms to solve the molecular mass of the compound. The mass is taken in grams because the atomic and molecular masses are in gram /mol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




