
Going from fluorine, chlorine, bromine to iodine the electronegativity:
A. Increases
B. First decreases then increases
C. Decreases
D. Changes randomly
Answer
582.6k+ views
Hint: Electronegativity is the tendency of an atom to attract a shared pair of electrons to itself. Size is a crucial factor in determining the electronegativity of an atom. There are some exceptions also which you have to remember.
Complete step by step solution:
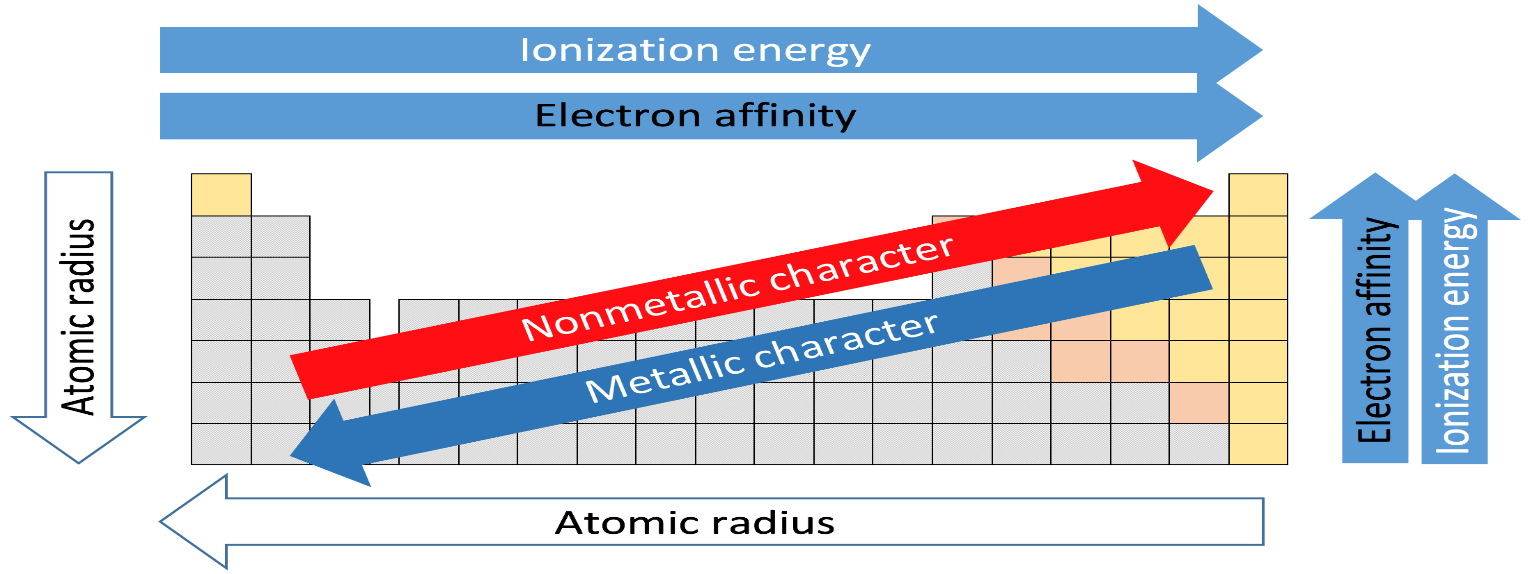
First let us discuss electronegativity and see which factors are responsible in determining the electronegativity.
Electronegativity is just a tendency of an atom to attract a shared pair of electrons towards itself. More the electronegativity of an atom , the more its electron attracts to itself. Electronegativity of an atom depends upon its ionization energy and its electron affinity.
Small atoms have higher electronegativity because the atoms which have nearly filled shells of electrons have higher electronegativity than those with less than half filled.
Factors affecting electronegativity:
1. Atomic Size: The smaller the size of an atom the greater its tendency to attract the shared pair of electrons. So smaller atoms have more electronegativity.
2. Number of inner shells: The atom with the greater number of inner shells has less value of electronegativity than the atom with smaller number of inner shells.
Now let's move to the question so we are going from top to bottom in a group and so size is increases and no. of inner shells also increases and moreover the nuclear charge is decreasing which results in the decrease in electronegativity.
So as we move down the group from fluorine, chlorine, bromine to iodine the electronegativity decreases.
Hence our correct option is C.
Note:
Nuclear charge decreases down the group due to no. of shells increases so its shielding effect on outer valence electrons decreases and hence electronegativity decreases.
Complete step by step solution:
First let us discuss electronegativity and see which factors are responsible in determining the electronegativity.
Electronegativity is just a tendency of an atom to attract a shared pair of electrons towards itself. More the electronegativity of an atom , the more its electron attracts to itself. Electronegativity of an atom depends upon its ionization energy and its electron affinity.
Small atoms have higher electronegativity because the atoms which have nearly filled shells of electrons have higher electronegativity than those with less than half filled.
Factors affecting electronegativity:
1. Atomic Size: The smaller the size of an atom the greater its tendency to attract the shared pair of electrons. So smaller atoms have more electronegativity.
2. Number of inner shells: The atom with the greater number of inner shells has less value of electronegativity than the atom with smaller number of inner shells.
Now let's move to the question so we are going from top to bottom in a group and so size is increases and no. of inner shells also increases and moreover the nuclear charge is decreasing which results in the decrease in electronegativity.
So as we move down the group from fluorine, chlorine, bromine to iodine the electronegativity decreases.

Hence our correct option is C.
Note:
Nuclear charge decreases down the group due to no. of shells increases so its shielding effect on outer valence electrons decreases and hence electronegativity decreases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




