
Glycol on heating with ${{P}}{{{I}}_3}$ mainly gives:
A. ethylene
B. ethylene iodide
C. ethyl iodide
D. ethane
Answer
544.8k+ views
Hint:Alcohols are the organic compounds having hydroxyl functional groups. Iodoform test is used to check the presence of carbonyl compounds or certain alcohols in an unknown compound. Iodoform test is used only for secondary alcohols with a methyl group in alpha position and ethanol.
Complete step by step answer:
Glycol has a chemical formula \[{{{C}}_2}{{{H}}_4}{{O}}\]. Its structure is given below:
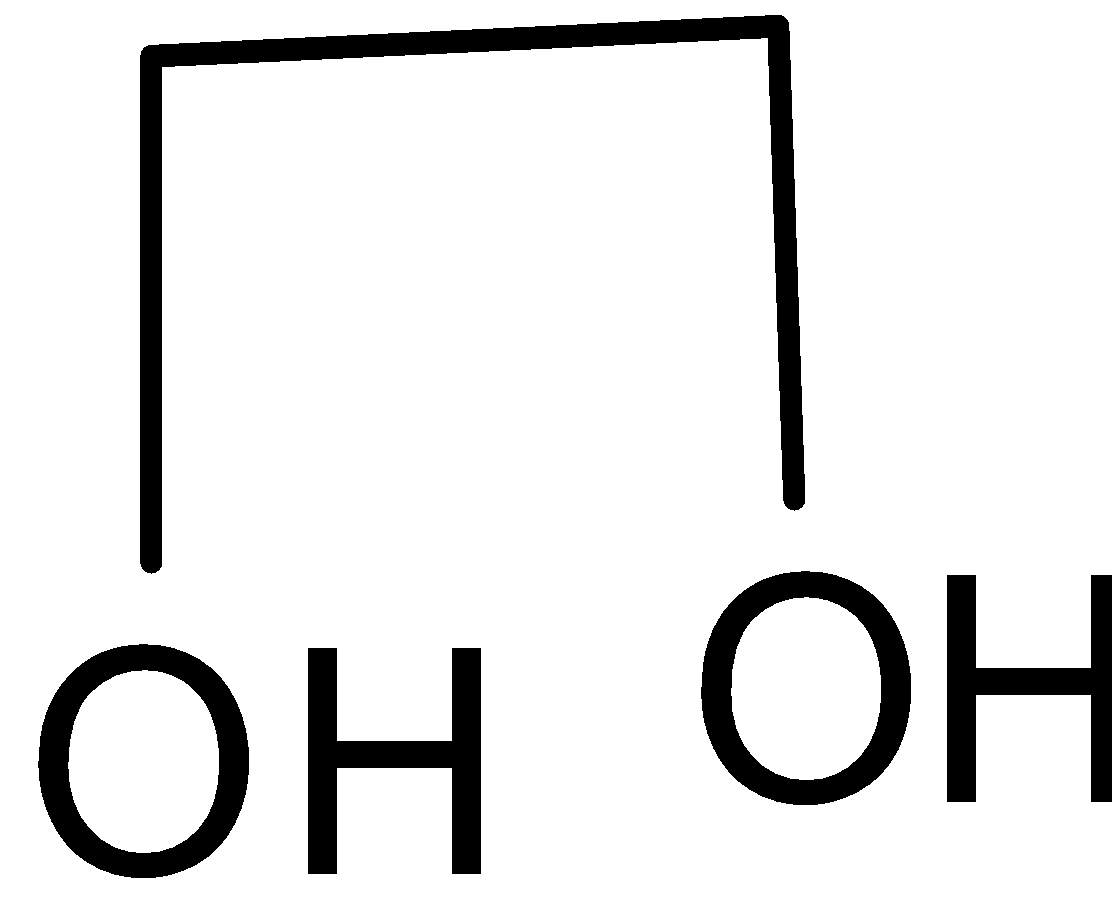
It has two hydroxyl groups, i.e. it is an alcohol. Haloform reaction is a chemical reaction where a haloform is produced by the exhaustive halogenation of aldehyde or ketone or certain alcohol in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups or to produce haloform. In iodoform test, triiodo methane is formed as a product.
Initially the alcohols are converted to aldehyde, then it is iodinated forming a yellow precipitate. Tertiary alcohols cannot be oxidized because it does not have any hydrogen atom attached to the carbon. Moreover only ethanol gives positive results for iodoform test among primary alcohols. Secondary alcohols can be oxidized to ketones, thereby giving results for iodoform test. Simple secondary alcohols with hydroxyl groups in the second carbon give positive results for iodoform test.
When glycol is heated with phosphorus triiodide, it forms ethylene diiodide initially. Then it loses its iodine and forms ethylene molecules.
Hence the correct option is A.
Note:
When ethylene glycol is reacted with ${{PC}}{{{l}}_3}$, it forms ethylene dichloride. But this does not happen when it is heated with ${{P}}{{{I}}_3}$. This is because ethylene diiodide is an unstable compound and it is also sterically hindered. Thus it breaks down into ethylene and iodide molecules when heated.
Complete step by step answer:
Glycol has a chemical formula \[{{{C}}_2}{{{H}}_4}{{O}}\]. Its structure is given below:

It has two hydroxyl groups, i.e. it is an alcohol. Haloform reaction is a chemical reaction where a haloform is produced by the exhaustive halogenation of aldehyde or ketone or certain alcohol in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups or to produce haloform. In iodoform test, triiodo methane is formed as a product.
Initially the alcohols are converted to aldehyde, then it is iodinated forming a yellow precipitate. Tertiary alcohols cannot be oxidized because it does not have any hydrogen atom attached to the carbon. Moreover only ethanol gives positive results for iodoform test among primary alcohols. Secondary alcohols can be oxidized to ketones, thereby giving results for iodoform test. Simple secondary alcohols with hydroxyl groups in the second carbon give positive results for iodoform test.
When glycol is heated with phosphorus triiodide, it forms ethylene diiodide initially. Then it loses its iodine and forms ethylene molecules.
Hence the correct option is A.
Note:
When ethylene glycol is reacted with ${{PC}}{{{l}}_3}$, it forms ethylene dichloride. But this does not happen when it is heated with ${{P}}{{{I}}_3}$. This is because ethylene diiodide is an unstable compound and it is also sterically hindered. Thus it breaks down into ethylene and iodide molecules when heated.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




