
Glucose on reaction with acetic acid gives glucose Penta acetate. What does it suggest about the structure of glucose?
Answer
481.5k+ views
Hint: Recall the reaction of glucose with acetic anhydride. It is the same reaction. Glucose with acetic anhydride also gives glucose Penta acetate. The reaction is known as the acetylation of glucose. The glucose Penta acetate is a very stable compound.
Complete Step By Step Answer:
The above question states that “Glucose on reaction with acetic acid gives glucose Penta acetate”. Now we will have to look into the reaction of glucose with Penta acetate to solve the problem and to find out the structure of glucose.
When glucose reacts with acetic acid it gives Penta acetate. The reaction takes place under the presence of Zinc chloride. The reaction is as follows:
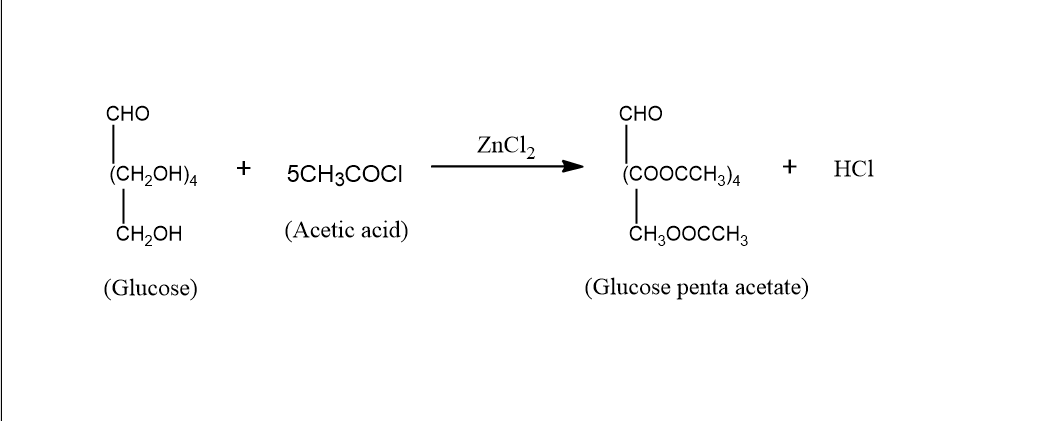
In the above reaction, we can see that the glucose structure has $5$OH groups in it. When these $5$ OH groups react with acetic acid the $5$OH groups of glucose get replaced by the acetate group. Thus, we can say that the reaction shows the presence of $5$OH groups in the structure of the glucose.
This glucose Penta acetate is the beta-D form of glucose. It is insoluble in water. It is soluble in chloroform and methanol. Its chemical stability is very high and has a long shelf life.
Note:
The above reaction is similar to that of the reaction of glucose with acetic anhydride. This is also produced by glucose Penta acetate and confirms the presence of $5$OH groups in glucose. This reaction is known as an acetylation reaction. The glucose Penta acetate is the protected form of glucose that is a key building block of any chemical synthesis of glucose-containing oligosaccharides or glycoconjugates.
Complete Step By Step Answer:
The above question states that “Glucose on reaction with acetic acid gives glucose Penta acetate”. Now we will have to look into the reaction of glucose with Penta acetate to solve the problem and to find out the structure of glucose.
When glucose reacts with acetic acid it gives Penta acetate. The reaction takes place under the presence of Zinc chloride. The reaction is as follows:

In the above reaction, we can see that the glucose structure has $5$OH groups in it. When these $5$ OH groups react with acetic acid the $5$OH groups of glucose get replaced by the acetate group. Thus, we can say that the reaction shows the presence of $5$OH groups in the structure of the glucose.
This glucose Penta acetate is the beta-D form of glucose. It is insoluble in water. It is soluble in chloroform and methanol. Its chemical stability is very high and has a long shelf life.
Note:
The above reaction is similar to that of the reaction of glucose with acetic anhydride. This is also produced by glucose Penta acetate and confirms the presence of $5$OH groups in glucose. This reaction is known as an acetylation reaction. The glucose Penta acetate is the protected form of glucose that is a key building block of any chemical synthesis of glucose-containing oligosaccharides or glycoconjugates.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




