
Glucose is oxidized by nitric acid and produce:
A.sorbitol
B.saccharic acid
C.furfural
D.glucuronic acid
Answer
575.4k+ views
Hint: We know that, glucose is an aldohexose whose chemical formula is ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}$. It is the monomer of many large carbohydrates such as starch, cellulose etc. Glucose exists in nature in either free or combined form.
Complete step by step answer:
Let’s understand glucose in detail. It is also known by the name dextrose. It is the most abundant organic compound on earth. In honey and sweet fruits glucose is present. In ripe grapes also, it is found in large amounts. The structure of glucose is,
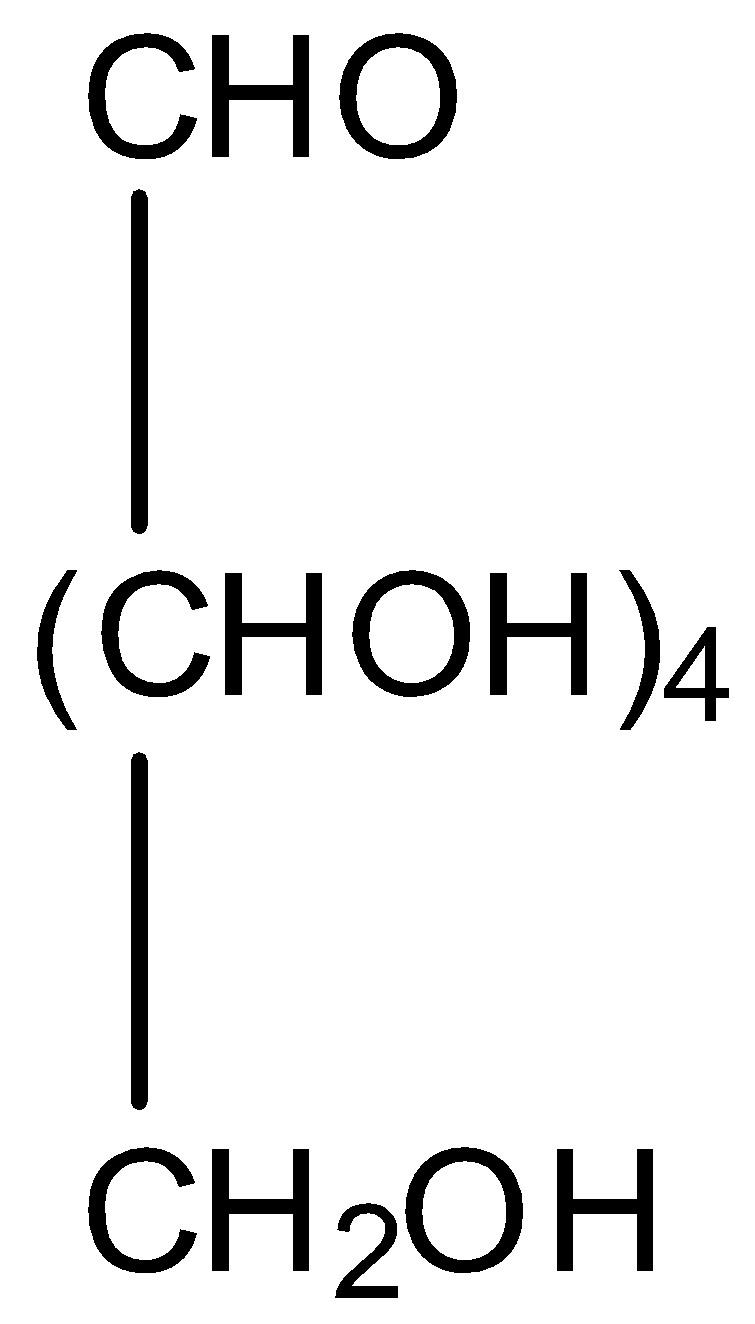
Now, come to the question. We are asked to identify the product of reaction of glucose with nitric acid. Nitric acid oxidizes glucose to saccharic acid. The reaction is,
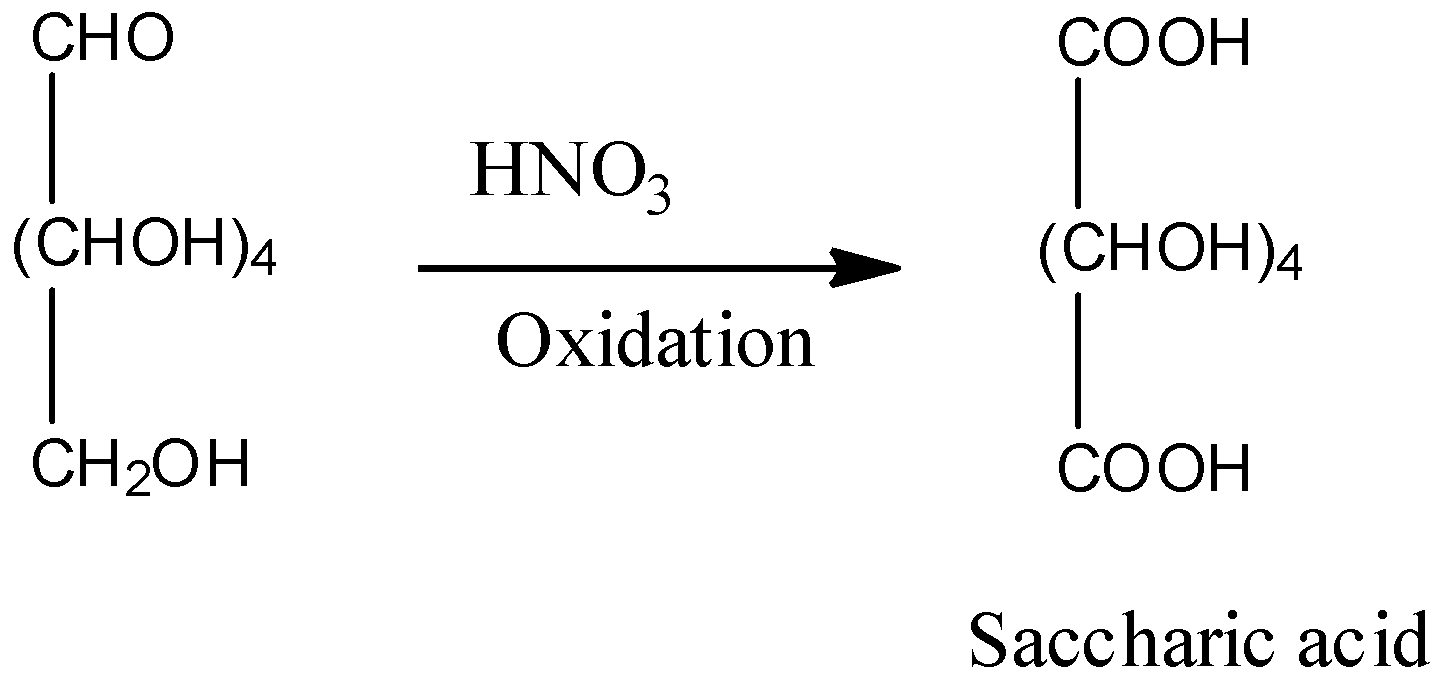
So, the correct answer is Option B.
Additional Information:
Let’s understand some chemical reactions of glucose.
1)Heating of glucose with HI for a long time produces n-hexane. This reaction gives the idea that all the carbon atoms of glucose exist in a straight chain.
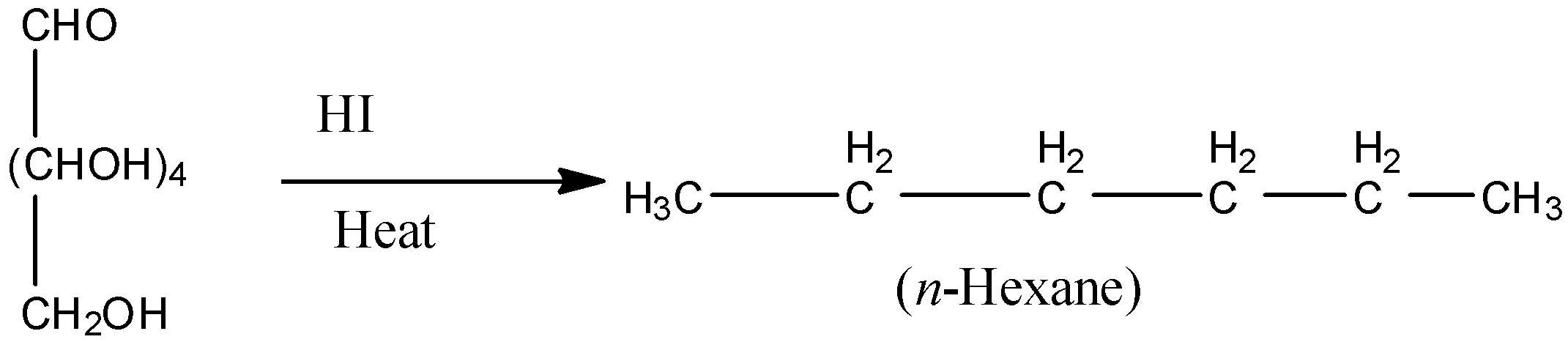
2)Reaction of glucose with hydroxylamine forms an oxime. Also addition of a HCN to glucose gives cyanohydrins. These reactions give an idea of the presence of carbonyl groups in the glucose.
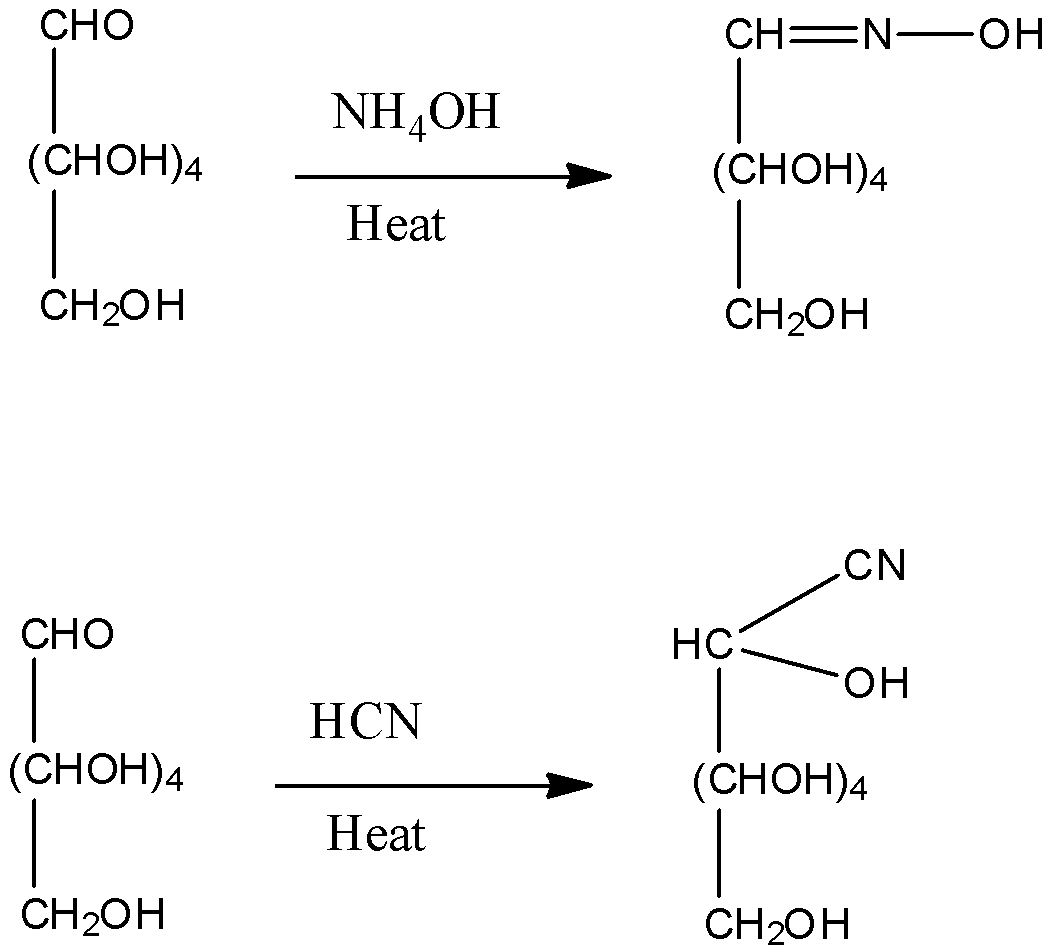
3)The oxidation of glucose is done by reaction glucose with ${\rm{B}}{{\rm{r}}_{\rm{2}}}$ water. Bromine water is an oxidizing agent. This reaction indicates the presence of the aldehyde group in the compound.
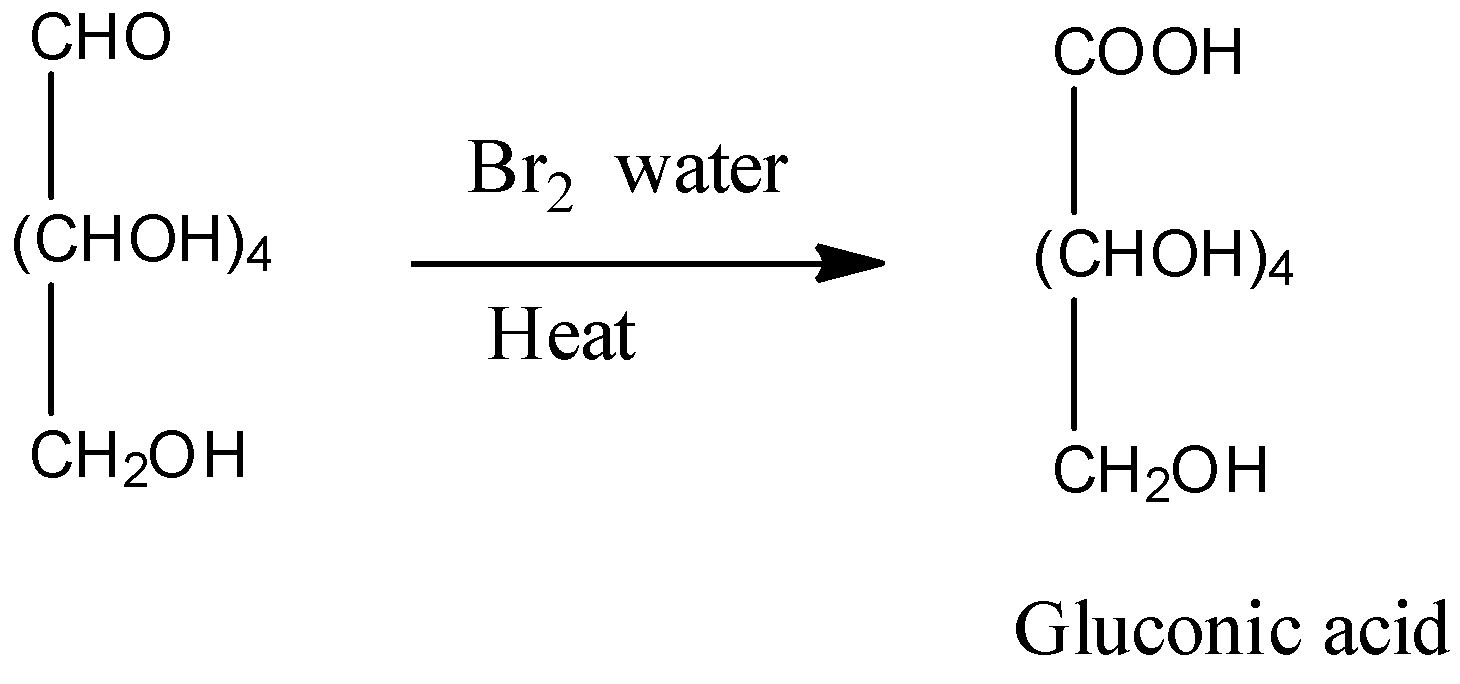
Note: There are two methods of preparation of glucose.
1)The boiling of sucrose with dilute HCl or sulphuric acid in alcoholic solution produces equal amounts of glucose and fructose.
$C_{12}H_{22}O_{11}+H_{2}O\overset{H^{+}}{\rightarrow}{C_{6}H_{12}O_6}+{C_{6}H_{12}O_6}$
2)Another method of production of glucose is by hydrolysis of starch by boiling it with dilute sulphuric acid at 393 K.
$\left ( C_{6}H_{10}O_{5} \right )_{n}+nH_{2}O\overset{H^{+}}{\rightarrow}nC_{6}H_{12}O_{6}$
Complete step by step answer:
Let’s understand glucose in detail. It is also known by the name dextrose. It is the most abundant organic compound on earth. In honey and sweet fruits glucose is present. In ripe grapes also, it is found in large amounts. The structure of glucose is,

Now, come to the question. We are asked to identify the product of reaction of glucose with nitric acid. Nitric acid oxidizes glucose to saccharic acid. The reaction is,

So, the correct answer is Option B.
Additional Information:
Let’s understand some chemical reactions of glucose.
1)Heating of glucose with HI for a long time produces n-hexane. This reaction gives the idea that all the carbon atoms of glucose exist in a straight chain.

2)Reaction of glucose with hydroxylamine forms an oxime. Also addition of a HCN to glucose gives cyanohydrins. These reactions give an idea of the presence of carbonyl groups in the glucose.

3)The oxidation of glucose is done by reaction glucose with ${\rm{B}}{{\rm{r}}_{\rm{2}}}$ water. Bromine water is an oxidizing agent. This reaction indicates the presence of the aldehyde group in the compound.

Note: There are two methods of preparation of glucose.
1)The boiling of sucrose with dilute HCl or sulphuric acid in alcoholic solution produces equal amounts of glucose and fructose.
$C_{12}H_{22}O_{11}+H_{2}O\overset{H^{+}}{\rightarrow}{C_{6}H_{12}O_6}+{C_{6}H_{12}O_6}$
2)Another method of production of glucose is by hydrolysis of starch by boiling it with dilute sulphuric acid at 393 K.
$\left ( C_{6}H_{10}O_{5} \right )_{n}+nH_{2}O\overset{H^{+}}{\rightarrow}nC_{6}H_{12}O_{6}$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




