
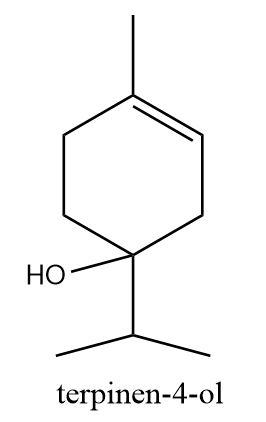
Given,\[Terpinen - 4 - ol\] is one of the active ingredients in tea tree oil. What is the molecular formula of \[Terpinen - 4 - ol\] ?
A. \[{C_7}{H_{11}}O\]
B. \[{C_{10}}{H_{16}}O\]
C. \[{C_{10}}{H_{17}}O\]
D. \[{C_{10}}{H_{18}}O\]

Answer
504.6k+ views
Hint: \[Terpinen - 4 - ol\] is a derivative of the naturally occurring organic compound ‘terpene’ and therefore its structure can be determined by placing the hydroxyl group at the forth position of the parent hydrocarbon chain.
Complete answer:
Terpenoids is a name given to a wide variety of open chain or cyclic organic compounds that contain one or more isoprene units which have a five carbon skeleton present in them. These compounds are mainly naturally occurring and have a characteristic fragrance or flavor due to which they are the major constituents of essential oils.
\[Terpinen - 4 - ol\] is an isomer of terpineol and a derivative of terpene. This volatile organic compound contains an alcohol functional group in addition to the core structure of terpenes.
The isoprene rule is used to count the number of isoprene units present in a terpene or terpene derivative connected in a head to tail arrangement. According to this rule, the terpene derivatives having the simplest structures are Monoterpenoids. These monoterpenoids are dimers of isoprene and contain a total of ten carbon atoms in their parent chain.
\[Terpinen - 4 - ol\] is a monoterpenoid with the hydroxyl (alcohol functional group) attached at the fourth position. Hence, the molecular formula of \[Terpinen - 4 - ol\] is :
\[{C_{10}}{H_{18}}O\]
So, the correct answer is “Option D”.
Note:
Most terpenes are characterized, numbered and named according to the isoprene rule which was postulated by Wallach. The rule however is not applicable to all isoprene compounds, some compounds like carotenes with a tail-tail linkage do not follow the rule.
Complete answer:
Terpenoids is a name given to a wide variety of open chain or cyclic organic compounds that contain one or more isoprene units which have a five carbon skeleton present in them. These compounds are mainly naturally occurring and have a characteristic fragrance or flavor due to which they are the major constituents of essential oils.
\[Terpinen - 4 - ol\] is an isomer of terpineol and a derivative of terpene. This volatile organic compound contains an alcohol functional group in addition to the core structure of terpenes.
The isoprene rule is used to count the number of isoprene units present in a terpene or terpene derivative connected in a head to tail arrangement. According to this rule, the terpene derivatives having the simplest structures are Monoterpenoids. These monoterpenoids are dimers of isoprene and contain a total of ten carbon atoms in their parent chain.
\[Terpinen - 4 - ol\] is a monoterpenoid with the hydroxyl (alcohol functional group) attached at the fourth position. Hence, the molecular formula of \[Terpinen - 4 - ol\] is :
\[{C_{10}}{H_{18}}O\]
So, the correct answer is “Option D”.
Note:
Most terpenes are characterized, numbered and named according to the isoprene rule which was postulated by Wallach. The rule however is not applicable to all isoprene compounds, some compounds like carotenes with a tail-tail linkage do not follow the rule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




