
Given,\[SO_4^{2 - } \to {S_2}O_8^{2 - } + 2{e^ - }\](ox.) How it is possible as there is no change in the oxidation state of \[S\] from sulphate ion to thionate i.e. +6 to +6
Answer
507.3k+ views
Hint: Check the structure of both \[SO_4^{2 - }\]and \[{S_2}O_8^{2 - }\] to know what the change of oxidation state is. \[{S_2}O_8^{2 - }\] has a peroxide linkage whereas \[SO_4^{2 - }\] doesn’t.
The structure of \[{S_2}O_8^{2 - }\] is given below:
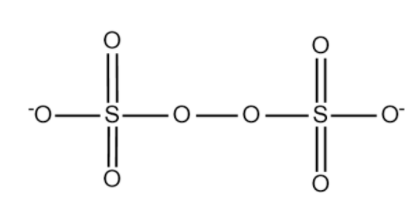
The structure of \[SO_4^{2 - }\] is given below:
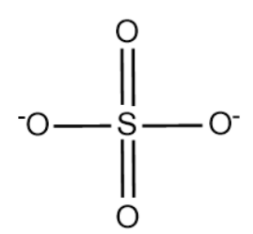
Complete answer:
Here we note that even though Sulphur doesn’t show any change in oxidation state, Oxygen does have a change. The peroxy bond in \[{S_2}O_8^{2 - }\] disappears when it becomes \[SO_4^{2 - }\]. While counting the oxidation number of sulphur in \[{S_2}O_8^{2 - }\] we consider the oxidation states of those oxygens (in peroxy bonds) to have -1 state. So from \[SO_4^{2 - }\], where those 2 oxygen atoms had -2 oxidation state, we have lost 2 electrons when the peroxy bond is formed and got \[{S_2}O_8^{2 - }\]. This means that there is net oxidation.
From -2 to -1 change in oxidation state means that there is net increase in oxidation state
This means oxidation
To summarise, change in oxidation is not due to the sulphur atom but due to the oxygen atom present in them. Sulphur oxidation state is +6 in both \[{S_2}O_8^{2 - }\] and \[SO_4^{2 - }\].
Note:
To calculate the Oxidation state of sulphur in \[{S_2}O_8^{2 - }\]
Total there are 8 oxygen atoms in which 2 forms peroxy bonds. So we have 6 oxygen atoms with -2 oxidation state and 2 oxygen atoms with -1 oxidation state.
So we can say that
\[ \Rightarrow 2 \times x + (2 \times - 1) + (6 \times - 2) = - 2\]
Where x is the oxidation state of sulphur
\[ \Rightarrow 2x - 2 - 12 = - 2\]
\[ \Rightarrow 2x - 14 = - 2\]
\[ \Rightarrow 2x = 12\]
\[ \Rightarrow x = 6\]
Hence we can say that oxidation state of sulphur is +6 in \[{S_2}O_8^{2 - }\]
To calculate the Oxidation state of sulphur in \[SO_4^{2 - }\]
Total 4 oxygen atom each having -2 oxidation number
\[ \Rightarrow x + (4 \times - 2) = - 2\]
Where x is the oxidation state of sulphur
\[ \Rightarrow x - 8 = - 2\]
\[ \Rightarrow x = 6\]
Hence we can say that oxidation state of sulphur is +6 in \[SO_4^{2 - }\]
The structure of \[{S_2}O_8^{2 - }\] is given below:

The structure of \[SO_4^{2 - }\] is given below:

Complete answer:
Here we note that even though Sulphur doesn’t show any change in oxidation state, Oxygen does have a change. The peroxy bond in \[{S_2}O_8^{2 - }\] disappears when it becomes \[SO_4^{2 - }\]. While counting the oxidation number of sulphur in \[{S_2}O_8^{2 - }\] we consider the oxidation states of those oxygens (in peroxy bonds) to have -1 state. So from \[SO_4^{2 - }\], where those 2 oxygen atoms had -2 oxidation state, we have lost 2 electrons when the peroxy bond is formed and got \[{S_2}O_8^{2 - }\]. This means that there is net oxidation.
From -2 to -1 change in oxidation state means that there is net increase in oxidation state
This means oxidation
To summarise, change in oxidation is not due to the sulphur atom but due to the oxygen atom present in them. Sulphur oxidation state is +6 in both \[{S_2}O_8^{2 - }\] and \[SO_4^{2 - }\].
Note:
To calculate the Oxidation state of sulphur in \[{S_2}O_8^{2 - }\]
Total there are 8 oxygen atoms in which 2 forms peroxy bonds. So we have 6 oxygen atoms with -2 oxidation state and 2 oxygen atoms with -1 oxidation state.
So we can say that
\[ \Rightarrow 2 \times x + (2 \times - 1) + (6 \times - 2) = - 2\]
Where x is the oxidation state of sulphur
\[ \Rightarrow 2x - 2 - 12 = - 2\]
\[ \Rightarrow 2x - 14 = - 2\]
\[ \Rightarrow 2x = 12\]
\[ \Rightarrow x = 6\]
Hence we can say that oxidation state of sulphur is +6 in \[{S_2}O_8^{2 - }\]
To calculate the Oxidation state of sulphur in \[SO_4^{2 - }\]
Total 4 oxygen atom each having -2 oxidation number
\[ \Rightarrow x + (4 \times - 2) = - 2\]
Where x is the oxidation state of sulphur
\[ \Rightarrow x - 8 = - 2\]
\[ \Rightarrow x = 6\]
Hence we can say that oxidation state of sulphur is +6 in \[SO_4^{2 - }\]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




