
Given,\[{C_2}{H_2}\](\[20\% {H_2}S{O_4},{\text{ }}1\% H{g^{2 + }}\]) =\[A\]. A (\[Zn - Pb/HCl\](concentrate)) =\[B\]. Identify the ‘\[A\]’ & ‘\[B\]’?
Answer
486k+ views
Hint: In organic chemistry, hydrocarbons are an important topic. Hydrocarbons are majorly classified into three groups. There are alkane, alkene and alkyne. The alkane means carbon-carbon single bond. The alkene having a carbon-carbon double bond. The alkyne means carbon-carbon having a triple bond in the molecule.
Complete answer:
The chemical name of \[{C_2}{H_2}\] is ethyne.
The molecular formula of ethyne is \[{C_2}{H_2}\].
The chemical structure of \[{C_2}{H_2}\] is
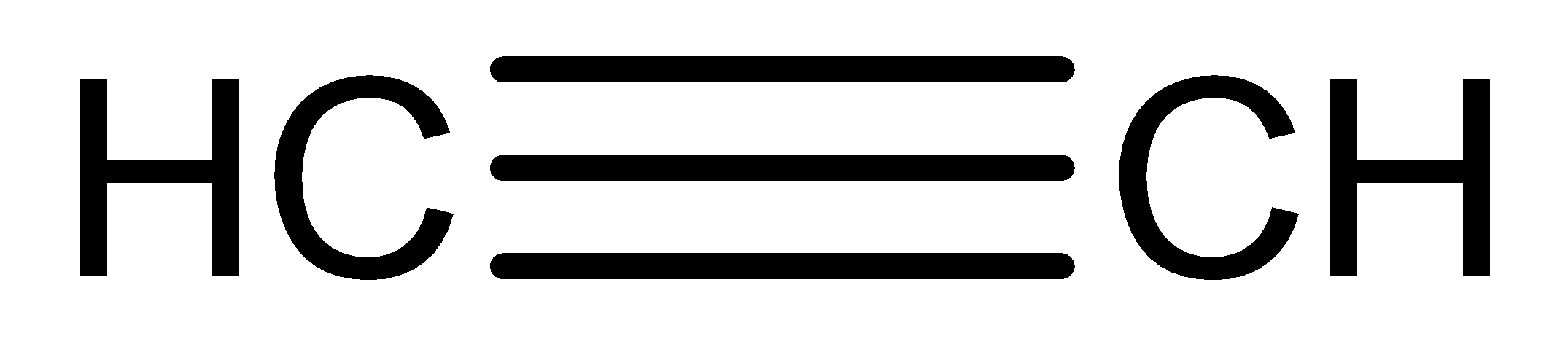
The ethyne is reacted with \[20\% {H_2}S{O_4},{\text{ }}1\% H{g^{2 + }}\] to form the product of ethanal.
The chemical reaction is given below,
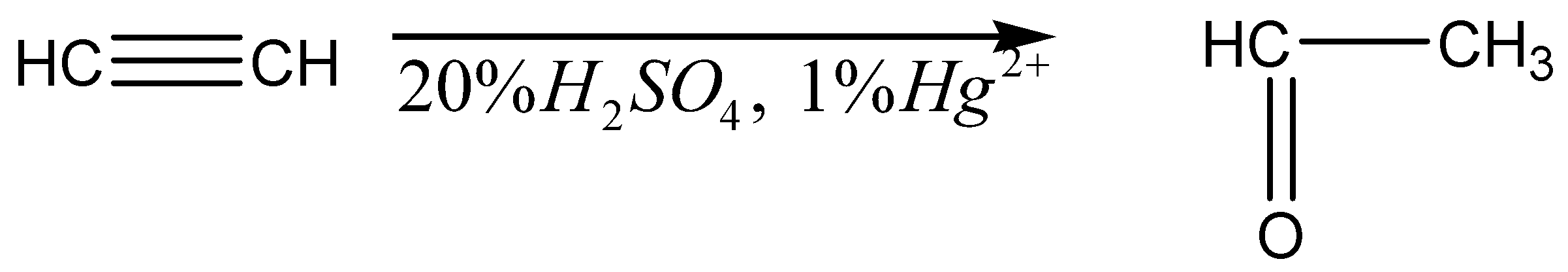
The ethanal is reacted with \[Zn - Pb/HCl\] to form the product of ethanol.
The chemical reaction for the above is given below,
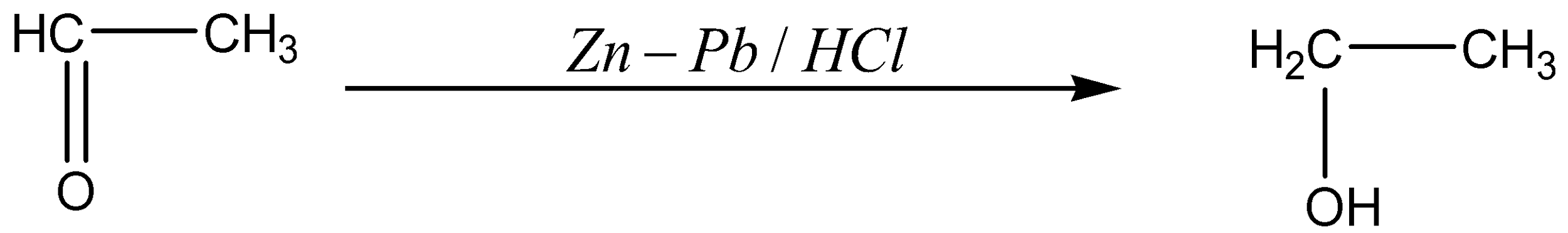
So, molecule \[A\] is ethanal and molecule \[B\] is ethanol.
According to the above, we conclude \[{C_2}{H_2}\] (\[20\% {H_2}S{O_4},{\text{ }}1\% H{g^{2 + }}\]) =. \[A\]\[A\] (\[Zn - Pb/HCl\] (concentrate)) = \[B\], molecule \[A\] is ethanal and molecule \[B\] is ethanol.
Note:
The conversion of one type of hydrocarbon to other hydrocarbons by oxidation and reduction. The oxidation of alkane gives an alkene. The oxidation of alkene gives alkyne. The reduction of alkyne to give an alkene. The reduction of alkene to give alkane. It having some general formula. \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n + 2}}}}\] is the general formula of an alkane. \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n}}}}\] is the general formula of an alkene. \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n - 2}}}}\] is the general formula of the alkyne. Oxidation means the addition of oxygen or removal of hydrogen or the loss of electrons in the reactant in the chemical reaction. The reduction means the removal of hydrogen or the addition of oxygen or the gain of electrons in the reaction in a chemical reaction.
Complete answer:
The chemical name of \[{C_2}{H_2}\] is ethyne.
The molecular formula of ethyne is \[{C_2}{H_2}\].
The chemical structure of \[{C_2}{H_2}\] is
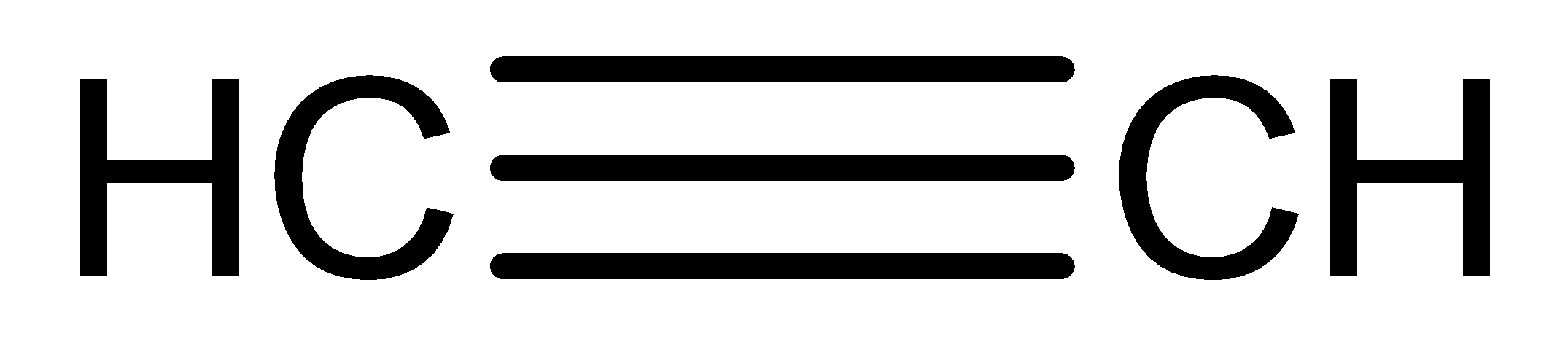
The ethyne is reacted with \[20\% {H_2}S{O_4},{\text{ }}1\% H{g^{2 + }}\] to form the product of ethanal.
The chemical reaction is given below,
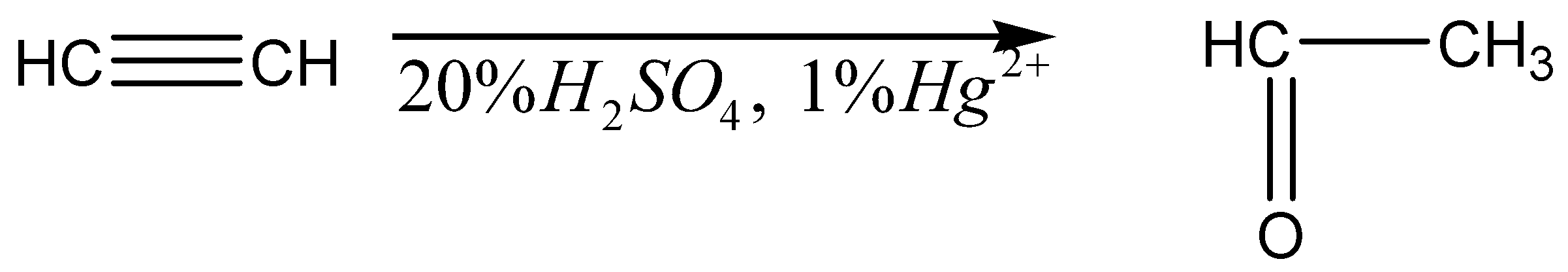
The ethanal is reacted with \[Zn - Pb/HCl\] to form the product of ethanol.
The chemical reaction for the above is given below,
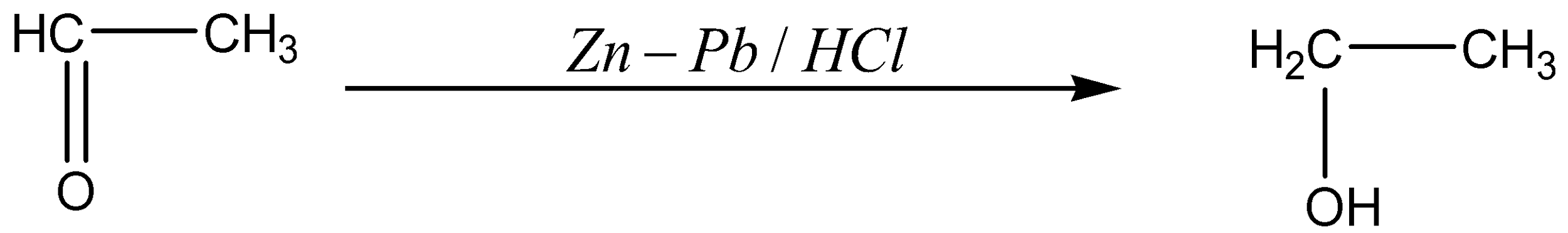
So, molecule \[A\] is ethanal and molecule \[B\] is ethanol.
According to the above, we conclude \[{C_2}{H_2}\] (\[20\% {H_2}S{O_4},{\text{ }}1\% H{g^{2 + }}\]) =. \[A\]\[A\] (\[Zn - Pb/HCl\] (concentrate)) = \[B\], molecule \[A\] is ethanal and molecule \[B\] is ethanol.
Note:
The conversion of one type of hydrocarbon to other hydrocarbons by oxidation and reduction. The oxidation of alkane gives an alkene. The oxidation of alkene gives alkyne. The reduction of alkyne to give an alkene. The reduction of alkene to give alkane. It having some general formula. \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n + 2}}}}\] is the general formula of an alkane. \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n}}}}\] is the general formula of an alkene. \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n - 2}}}}\] is the general formula of the alkyne. Oxidation means the addition of oxygen or removal of hydrogen or the loss of electrons in the reactant in the chemical reaction. The reduction means the removal of hydrogen or the addition of oxygen or the gain of electrons in the reaction in a chemical reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




