
Given,\[1,3 - \] butadiene has
A.sp and \[s{p^2}\] hybridized C-atoms
B.sp, \[s{p^2}\] and \[s{p^3}\] hybridized C-atoms
C.only \[s{p^2}\] hybridized C-atoms
D.only sp hybridized C-atoms
Answer
495.6k+ views
Hint: We need to know that hybridization is the method of mixing two atomic orbitals having the same energy levels, and there is a formation of degenerated new molecular orbitals. It is explained on the basis of quantum mechanics. In the time of hybridization, the atomic orbitals with similar energy will be mixed. In the case of \[s{p^2}\] –orbitals, one s-orbital and two p-orbitals are mixing.
Complete answer:
The \[1,3 - \]butadiene has only \[s{p^2}\] hybridized C-atoms. \[1,3 - \]butadiene is a chemical compound having the molecular formula \[C{H_2} - C{H_2} - C{H_2} - C{H_2}\]. And here all carbon atoms have \[s{p^2}\] hybridization. Let’s see the structure,
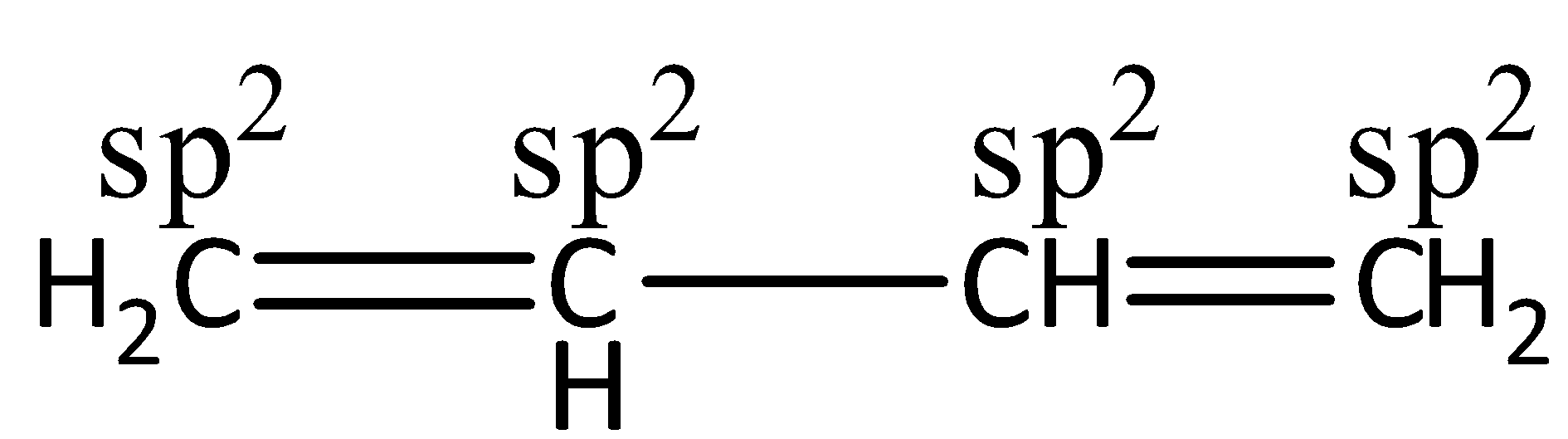
The hybridization is used for the explanation of molecular geometry. And the VSEPR theory is used for the prediction of geometry. The VSEPR theory is also known as Valence shell electron pair repulsion theory. It defines the geometry of individual molecules by using the number of pairs of electrons present around the central atoms. For example, for sp hybridization, the shape is linear, \[s{p^2} \to \] trigonal planar, \[s{p^3} \to \] tetrahedral, \[s{p^3}d \to \] trigonal pyramidal and \[s{p^3}{d^2} \to \] octahedral. Hence, we can say that the definite shape of the compound can be found by the combination of VSEPR theory and hybridization.
Note:
We need to know that the hybridization of orbital hybridization is a method of mixing atomic orbital with new hybrid orbitals having different shapes, energy than the atomic orbital components. In the case of \[s{p^2}\] and \[s{p^3}\] hybridization expresses the number of s orbital and p orbital and there is a formation of new degenerate hybrid orbitals. And hybridization is related with molecular geometry.
Complete answer:
The \[1,3 - \]butadiene has only \[s{p^2}\] hybridized C-atoms. \[1,3 - \]butadiene is a chemical compound having the molecular formula \[C{H_2} - C{H_2} - C{H_2} - C{H_2}\]. And here all carbon atoms have \[s{p^2}\] hybridization. Let’s see the structure,
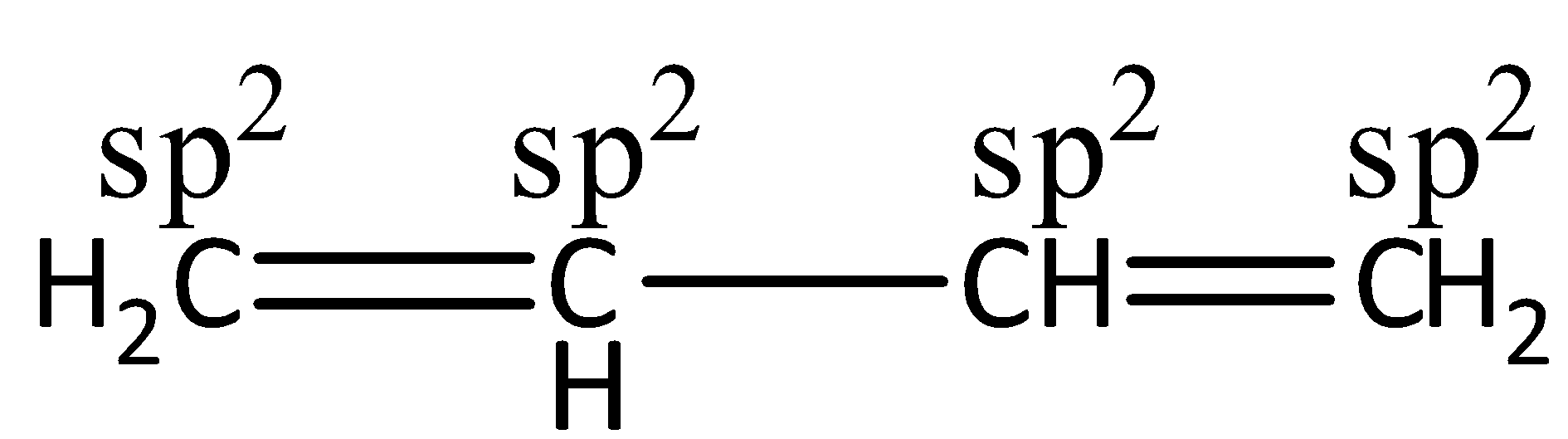
The hybridization is used for the explanation of molecular geometry. And the VSEPR theory is used for the prediction of geometry. The VSEPR theory is also known as Valence shell electron pair repulsion theory. It defines the geometry of individual molecules by using the number of pairs of electrons present around the central atoms. For example, for sp hybridization, the shape is linear, \[s{p^2} \to \] trigonal planar, \[s{p^3} \to \] tetrahedral, \[s{p^3}d \to \] trigonal pyramidal and \[s{p^3}{d^2} \to \] octahedral. Hence, we can say that the definite shape of the compound can be found by the combination of VSEPR theory and hybridization.
Note:
We need to know that the hybridization of orbital hybridization is a method of mixing atomic orbital with new hybrid orbitals having different shapes, energy than the atomic orbital components. In the case of \[s{p^2}\] and \[s{p^3}\] hybridization expresses the number of s orbital and p orbital and there is a formation of new degenerate hybrid orbitals. And hybridization is related with molecular geometry.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




