
Given, $Sb{{F}_{5}}$ reacts with $Xe{{F}_{4}}$ and $Xe{{F}_{6}}$ to form ionic compounds $[Sb{{F}_{6}}^{-}][Xe{{F}_{3}}^{+}]$ and $[Sb{{F}_{6}}^{-}][Xe{{F}_{5}}^{+}]$ respectively. The geometry of $Xe{{F}_{3}}^{+}$ ion and $Xe{{F}_{5}}^{+}$ ion respectively is:
A. square pyramidal, t-shaped
B. bent T-shaped, square pyramidal
C. see-saw, square pyramidal
D. square pyramidal, see-saw
Answer
592.5k+ views
Hint: Take note of the oxidation state of the $Xe$ atom and then consider the electronic configuration. From this, calculate the number of lone pairs and bond pairs. This will give you an idea about the hybridization as well as the geometry of the molecule.
Complete solution:
First, let us consider the electronic configuration of $Xe$ in its ground state. The atomic number of xenon is 54. Its electronic configuration is $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$. Usually, it is inert, but it can form bonds with different atoms by promoting its electrons to the $5d$ orbital and hybridization.
Both the ions that need to be considered here have a +1 charge, since fluorine cannot have lost one of its electrons, let us assume that it is xenon that has lost one. So, the configuration will be $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{5}}$. While drawing the electronic configuration, let us ignore the $4d$ orbital and only consider the $5s,\text{ }5p,\text{ and 5d}$ orbitals for the time being.
So, the configuration will be:
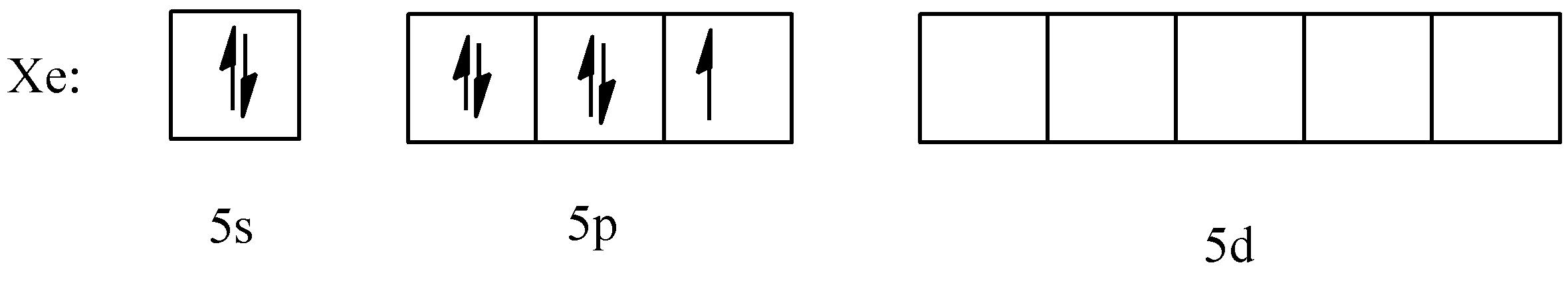
- For $Xe{{F}_{3}}^{+}$
To form bonds with 3 fluorine atoms, xenon will require 2 more free electrons, so it will promote one of the electrons from the $5p$ orbital to the $5d$ orbital and carry out the hybridization to form 5 $s{{p}^{3}}d$ orbitals. The hybridized configuration will be:
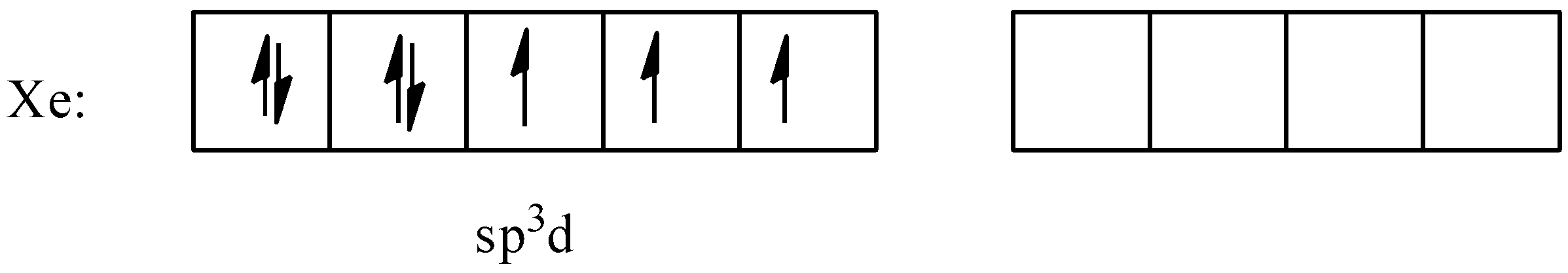
Now the three electrons will form bonds with 3 fluorine atoms and the geometry of the molecule will become trigonal bipyramidal. There are 2 lone pairs and 3 bond pairs present; the lone pairs always occupy the equatorial positions in the trigonal bipyramidal geometry. Thus, the shape of the molecule will be:
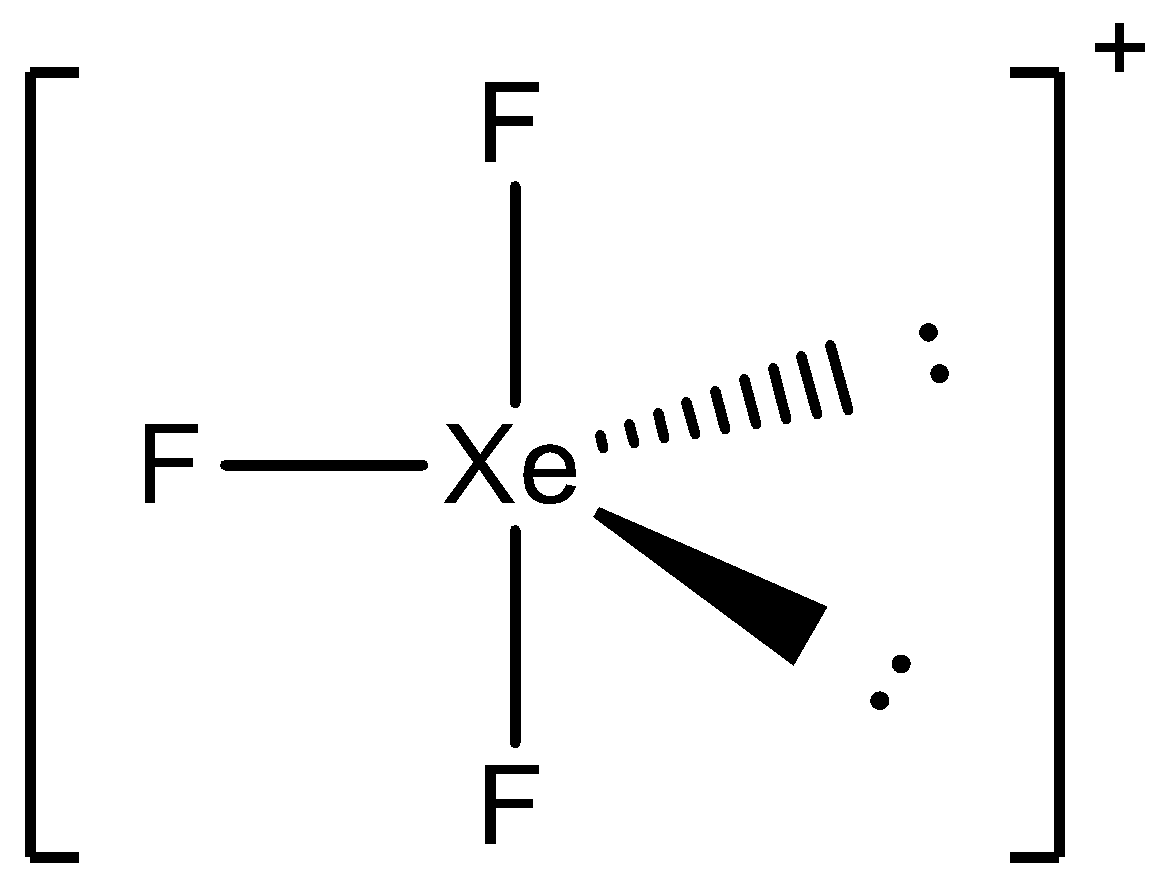
Here, we can see the bent T-shape formed by the atoms.
- For $Xe{{F}_{5}}^{+}$
The formation of this ion will be similar to that of the $Xe{{F}_{3}}^{+}$ ion. Since, xenon has to form bonds with 5 fluorine atoms, it will need 5 free electrons, so it will promote 2 electrons from the $5p$ orbital to the $5d$ orbital. And carry out the hybridization to form 6 $s{{p}^{3}}{{d}^{2}}$ orbitals. The hybridized configuration will be:
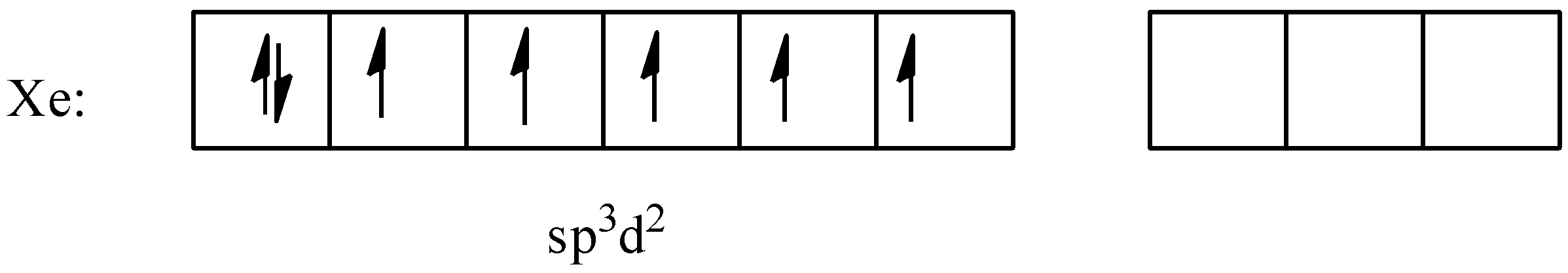
Now, the unpaired electrons will pair with the 5 fluorine atoms and the molecule will become octahedral. There are 5 bond pairs and 1 lone pair present. The geometry will be:
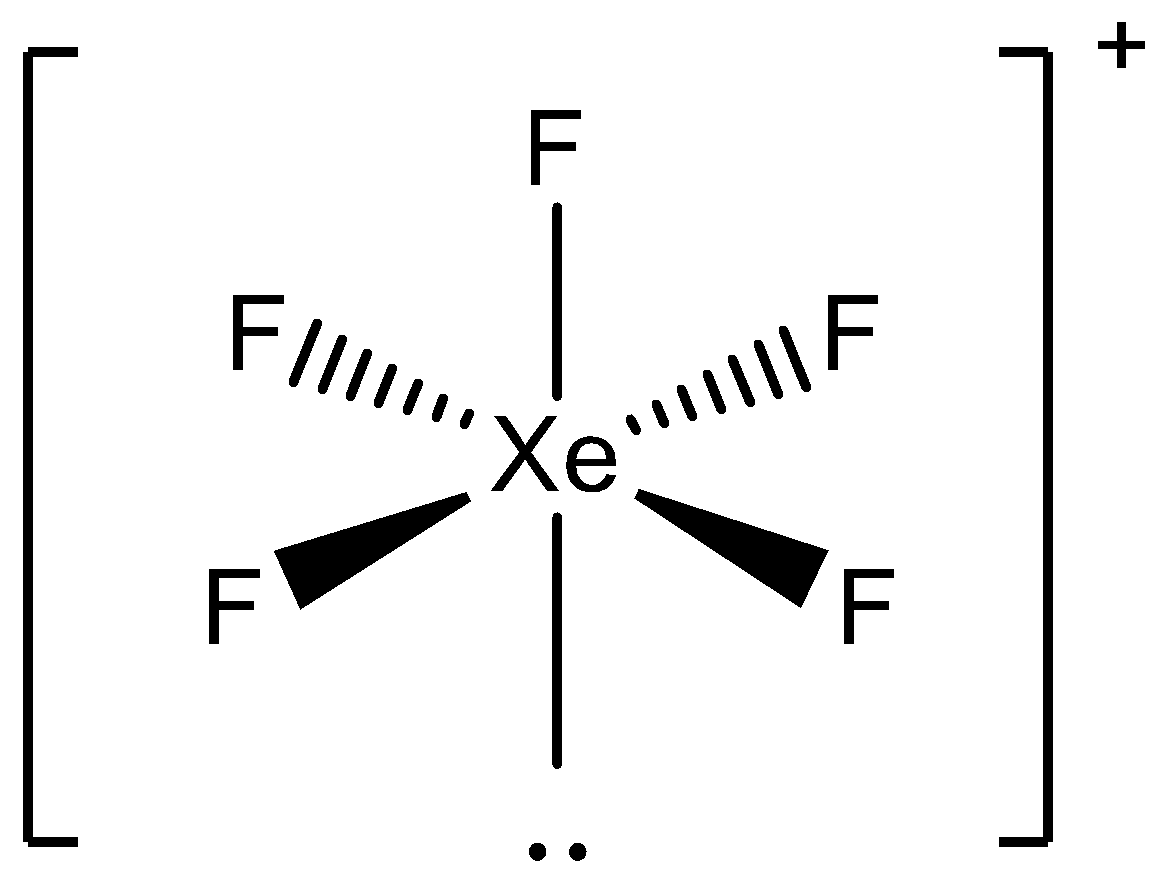
Here, we can see the square pyramid formed by the atoms.
The geometries of the $Xe{{F}_{3}}^{+}$ ion and $Xe{{F}_{5}}^{+}$ ion respectively is bent T-shaped and square pyramidal respectively.
Hence, the correct answer is ‘B. bent T-shaped, square pyramidal’
Note:
Always consider the charge that is present on the ions, if the charge is ignored then it will not be possible to draw the hybridization diagram. When xenon has to bond with an odd number of atoms, it always has to have a charge. Otherwise, one unpaired electron will remain and make the molecule unstable.
Complete solution:
First, let us consider the electronic configuration of $Xe$ in its ground state. The atomic number of xenon is 54. Its electronic configuration is $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$. Usually, it is inert, but it can form bonds with different atoms by promoting its electrons to the $5d$ orbital and hybridization.
Both the ions that need to be considered here have a +1 charge, since fluorine cannot have lost one of its electrons, let us assume that it is xenon that has lost one. So, the configuration will be $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{5}}$. While drawing the electronic configuration, let us ignore the $4d$ orbital and only consider the $5s,\text{ }5p,\text{ and 5d}$ orbitals for the time being.
So, the configuration will be:

- For $Xe{{F}_{3}}^{+}$
To form bonds with 3 fluorine atoms, xenon will require 2 more free electrons, so it will promote one of the electrons from the $5p$ orbital to the $5d$ orbital and carry out the hybridization to form 5 $s{{p}^{3}}d$ orbitals. The hybridized configuration will be:

Now the three electrons will form bonds with 3 fluorine atoms and the geometry of the molecule will become trigonal bipyramidal. There are 2 lone pairs and 3 bond pairs present; the lone pairs always occupy the equatorial positions in the trigonal bipyramidal geometry. Thus, the shape of the molecule will be:

Here, we can see the bent T-shape formed by the atoms.
- For $Xe{{F}_{5}}^{+}$
The formation of this ion will be similar to that of the $Xe{{F}_{3}}^{+}$ ion. Since, xenon has to form bonds with 5 fluorine atoms, it will need 5 free electrons, so it will promote 2 electrons from the $5p$ orbital to the $5d$ orbital. And carry out the hybridization to form 6 $s{{p}^{3}}{{d}^{2}}$ orbitals. The hybridized configuration will be:

Now, the unpaired electrons will pair with the 5 fluorine atoms and the molecule will become octahedral. There are 5 bond pairs and 1 lone pair present. The geometry will be:

Here, we can see the square pyramid formed by the atoms.
The geometries of the $Xe{{F}_{3}}^{+}$ ion and $Xe{{F}_{5}}^{+}$ ion respectively is bent T-shaped and square pyramidal respectively.
Hence, the correct answer is ‘B. bent T-shaped, square pyramidal’
Note:
Always consider the charge that is present on the ions, if the charge is ignored then it will not be possible to draw the hybridization diagram. When xenon has to bond with an odd number of atoms, it always has to have a charge. Otherwise, one unpaired electron will remain and make the molecule unstable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




