
Given,
$Phenol\xrightarrow[Distillation]{Zn}\text{ }A\xrightarrow[Conc.{{H}_{2}}S{{O}_{4}}]{Conc.HN{{O}_{3}}}\text{ }B\xrightarrow[NaOH]{Zn}\text{ }C$
In the above reaction sequence, A, B and C respectively are:
Options are-
(A) Benzene, nitrobenzene, aniline
(B) Benzene, m-dinitrobenzene, m-nitroaniline
(C) toluene, m-nitrotoluene ,m-toluidine
(D) benzene, nitrobenzene, hydra azobenzene
Answer
584.1k+ views
Hint: Metallic zinc is used in organic reactions to reduce the hydroxyl group attached to the carbon and replace it with hydrogen atom. Mixtures of Nitric acid and sulfuric acid are used to add a nitro group to the benzene ring and Zn in presence of NaOH partially reduces the nitro group present on the benzene ring.
Complete answer:
Let us write the first reaction to find the product A.
The vapours of phenol when passed through zinc dust, it loses the hydroxyl functional group giving benzene as the product. The reaction is given below:
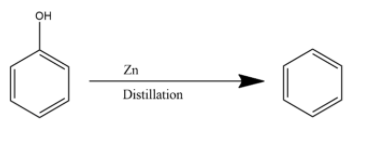
Hence the product (A) is Benzene.
Now let us write the product for the second reaction to find the product B.
Mixture of conc$HN{{O}_{3}}$and conc${{H}_{2}}S{{O}_{4}}$ is commonly referred to as the nitrating mixture. Nitrating mixture is used to attach a nitro group to the benzene ring. The reaction is given below:
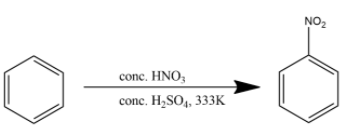
Hence the product (B) is nitrobenzene.
Let us now find the final product of the reaction i.e. C.
Zinc metal when combined with sodium hydroxide is used to reduce organic compounds. However, it cannot completely reduce the nitro functional group to an amine functional group. This is the reason two molecules of nitrobenzene react with Zn/NaOH to give hydra azobenzene. The reaction is given below:
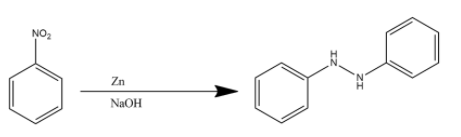
Hence the product (C) is hydra azobenzene.
Therefore, the correct answer is option (D).
Note:
When nitrating mixture is added to a derivative of benzene, the nitro group attached to either the para or ortho position of the dominant functional group.
However, nitration of benzene or its derivatives is not part of electrophilic aromatic substitution, as the nitrating mixture does not increase the nucleophilicity of the benzene ring which is a characteristic feature of EAS electrophiles.
Complete answer:
Let us write the first reaction to find the product A.
The vapours of phenol when passed through zinc dust, it loses the hydroxyl functional group giving benzene as the product. The reaction is given below:

Hence the product (A) is Benzene.
Now let us write the product for the second reaction to find the product B.
Mixture of conc$HN{{O}_{3}}$and conc${{H}_{2}}S{{O}_{4}}$ is commonly referred to as the nitrating mixture. Nitrating mixture is used to attach a nitro group to the benzene ring. The reaction is given below:

Hence the product (B) is nitrobenzene.
Let us now find the final product of the reaction i.e. C.
Zinc metal when combined with sodium hydroxide is used to reduce organic compounds. However, it cannot completely reduce the nitro functional group to an amine functional group. This is the reason two molecules of nitrobenzene react with Zn/NaOH to give hydra azobenzene. The reaction is given below:

Hence the product (C) is hydra azobenzene.
Therefore, the correct answer is option (D).
Note:
When nitrating mixture is added to a derivative of benzene, the nitro group attached to either the para or ortho position of the dominant functional group.
However, nitration of benzene or its derivatives is not part of electrophilic aromatic substitution, as the nitrating mixture does not increase the nucleophilicity of the benzene ring which is a characteristic feature of EAS electrophiles.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




