
Give the structure of Propanamide.
Answer
524.1k+ views
Hint: The chemical formula for propanamide is \[C{H_3}C{H_2}C = O(N{H_2}).\] That is propanoic acid's amide. A mono-substituted amide is this organic compound. Amide-group organic compounds can react in a variety of organic processes to produce other useful compounds for synthesis. The formal condensation of propionic acid with ammonia yields propanamide, a monocarboxylic acid amide. It's a main fatty amide and a monocarboxylic acid amide.
Complete answer: The chemical formula for propanamide is \[C{H_3}C{H_2}C = O(N{H_2}).\]That is propanoic acid's amide. A mono-substituted amide is this organic compound. Amide-group organic compounds can react in a variety of organic processes to produce other useful compounds for synthesis. The formal condensation of propionic acid with ammonia yields propanamide, a monocarboxylic acid amide. It's a main fatty amide and a monocarboxylic acid amide.
The condensation reaction between urea and propanoic acid will produce propanamide.
\[{(N{H_2})_2}CO + 2C{H_3}C{H_2}COOH \to C{H_3}C{H_2}CO(N{H_2}) + {H_2}O + C{O_2}\]
Structure of Propanamide =
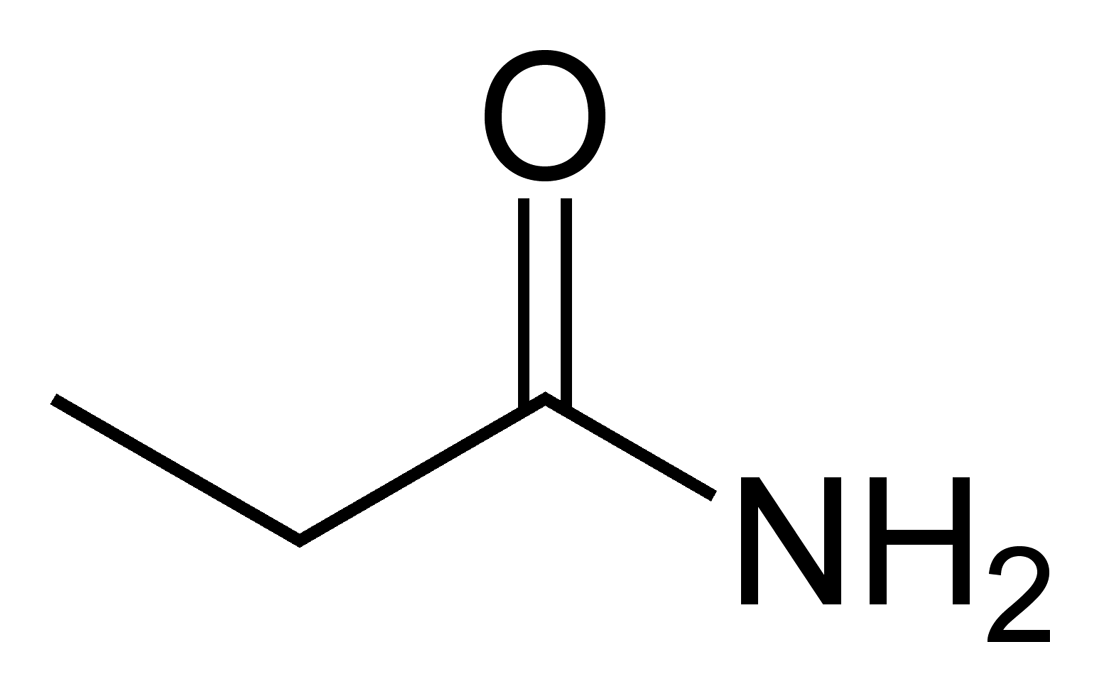
The organic compound urea, also known as carbamide, has the chemical formula \[CO{\left( {N{H_2}} \right)_2}.\]Two \[-N{H_2}\] groups are united by a carbonyl functional group in this amide. Urea is the primary nitrogen-containing agent in mammalian urine and plays a vital function in the metabolism of nitrogen-containing compounds by animals.
Propionic acid is a carboxylic acid that occurs naturally and has the chemical formula
\[C{H_3}C{H_2}COOH\]. It's a substance with a pungent, irritating odour that's similar to body odour.
The adsorption isotherms of acetamide and propionamide on multi-wall carbon nanotubes were determined using propionamide as an adsorbent. It was used in a reliable screening procedure to investigate biotransformations involving (+)-$\gamma$-lactamase.
Note:
The adsorption isotherms of acetamide and propionamide on multi-wall carbon nanotubes were determined using propionamide as an adsorbent. It was used in a reliable screening procedure to investigate biotransformations involving (+)-$\gamma$-lactamase.
Complete answer: The chemical formula for propanamide is \[C{H_3}C{H_2}C = O(N{H_2}).\]That is propanoic acid's amide. A mono-substituted amide is this organic compound. Amide-group organic compounds can react in a variety of organic processes to produce other useful compounds for synthesis. The formal condensation of propionic acid with ammonia yields propanamide, a monocarboxylic acid amide. It's a main fatty amide and a monocarboxylic acid amide.
The condensation reaction between urea and propanoic acid will produce propanamide.
\[{(N{H_2})_2}CO + 2C{H_3}C{H_2}COOH \to C{H_3}C{H_2}CO(N{H_2}) + {H_2}O + C{O_2}\]
Structure of Propanamide =

The organic compound urea, also known as carbamide, has the chemical formula \[CO{\left( {N{H_2}} \right)_2}.\]Two \[-N{H_2}\] groups are united by a carbonyl functional group in this amide. Urea is the primary nitrogen-containing agent in mammalian urine and plays a vital function in the metabolism of nitrogen-containing compounds by animals.
Propionic acid is a carboxylic acid that occurs naturally and has the chemical formula
\[C{H_3}C{H_2}COOH\]. It's a substance with a pungent, irritating odour that's similar to body odour.
The adsorption isotherms of acetamide and propionamide on multi-wall carbon nanotubes were determined using propionamide as an adsorbent. It was used in a reliable screening procedure to investigate biotransformations involving (+)-$\gamma$-lactamase.
Note:
The adsorption isotherms of acetamide and propionamide on multi-wall carbon nanotubes were determined using propionamide as an adsorbent. It was used in a reliable screening procedure to investigate biotransformations involving (+)-$\gamma$-lactamase.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE

Explain sex determination in humans with line diag class 12 biology CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE




