
Give the structure and names of the following structures:
TNT
Answer
573.9k+ views
Hint: Organic compounds usually have an IUPAC name and a common name used by experts in an industry. IUPAC names are characteristic for a particular compound and it reveals the properties, the class to which the compound belongs etc.by the name. Common names make it easier for the people working in the industry to easily identify the compound.
Complete step by step answer:
The naming or nomenclature of aromatic compounds deserve special attention. The term aromatic is derived from Greek word aroma which means pleasant smelling compounds. But in present form, aromatic compounds are cyclic compounds which contain one or more benzene type rings.
Benzene can undergo substitutions at its ortho, para and meta positions. Therefore, it can form three substituted derivatives.
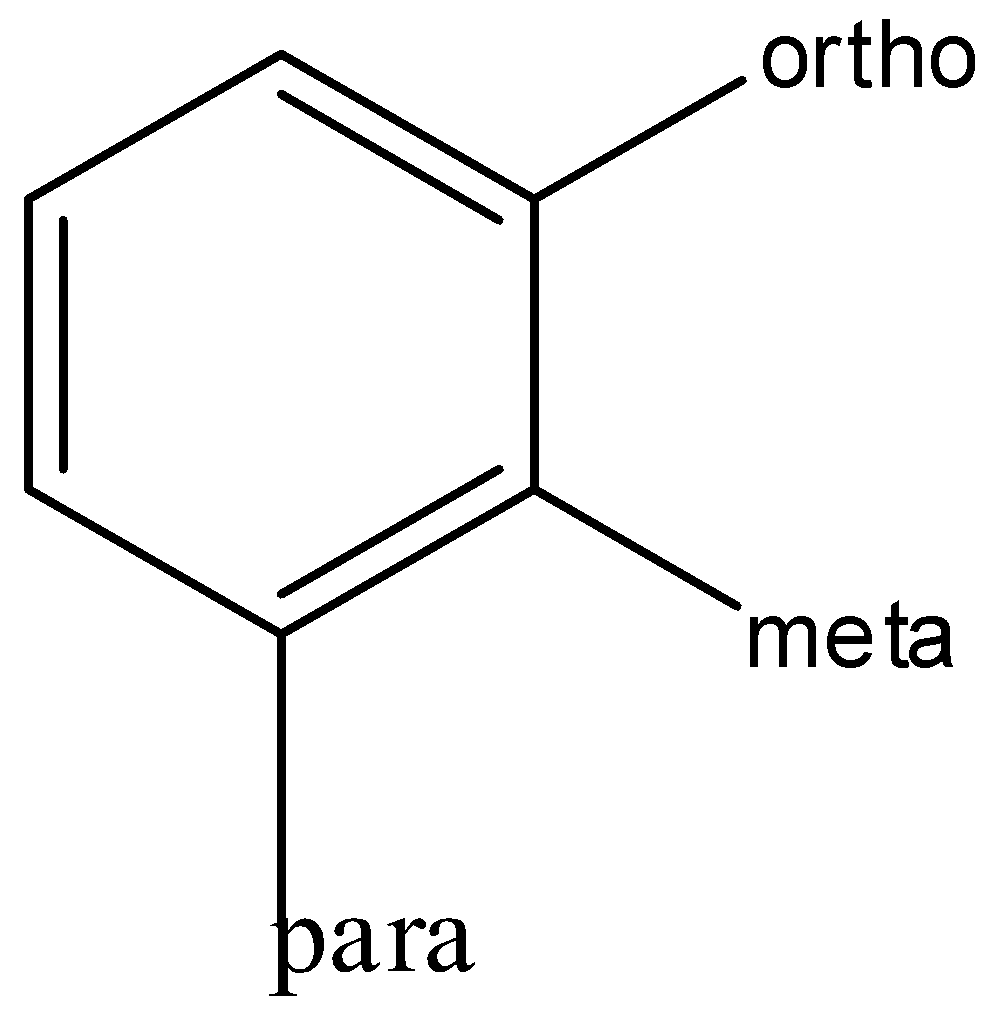
These are respectively called ortho, meta or para derivatives.
TNT or 2,4,6-Trinitrotoluene contains a tri-substituted benzene ring with a methyl group where the-\[{\text{N}}{{\text{O}}_{\text{2}}}\] groups occupy the two ortho and one para positions. The structure of the compound will be as follows;
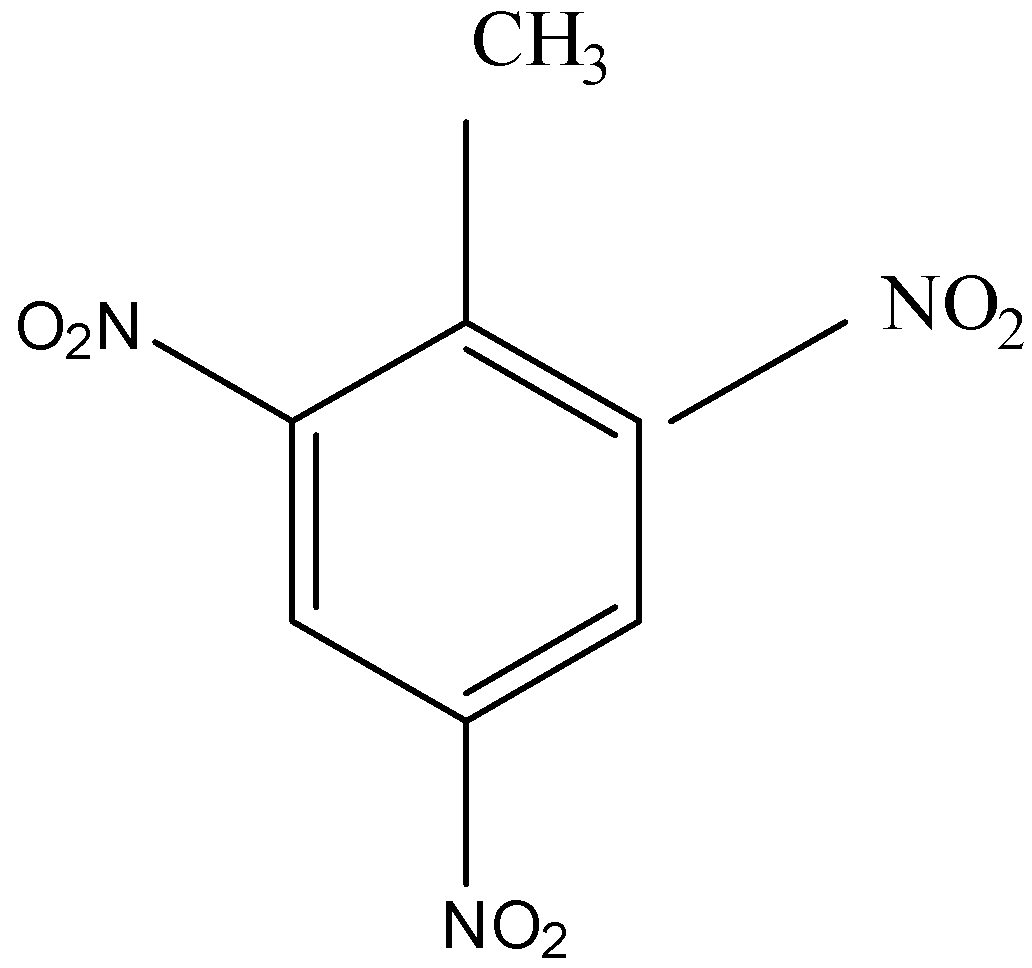
Tri and poly substituted derivatives are named by numbering the chain in such a way that the parent group gets the lowest number and sum of the positions of the substituents are the smallest. So, the compound is named as 2,4,6-Trinitrotoluene and has the molecular formula as \[{{\text{C}}_{\text{7}}}{{\text{H}}_{\text{5}}}{{\text{N}}_{\text{3}}}{{\text{O}}_{\text{6}}}\] \[\left( {{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{2}}}\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right){{\left( {{\text{N}}{{\text{O}}_{\text{2}}}} \right)}_{\text{3}}}} \right){\text{.}}\]
This compound is prepared by the continuous nitration toluene, ( \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{C}}{{\text{H}}_{\text{3}}}\]) and then it is crystallized from dilute nitric acid.
Additional Information:
TNT is a solid organic nitrogen compound which is mainly used as an explosive. It was widely used by the militaries of different countries during war time. It can explode under intense heat conditions.
Note: TNT is most favoured as an explosive and is extensively used in demolitions and munitions. It can also be used as an intermediate in the production of dyes and photographic chemicals. It is prepared by treating toluene with nitric acid continuously until the three positions in the benzene ring will be substituted by the- $NO_2$ groups.
Complete step by step answer:
The naming or nomenclature of aromatic compounds deserve special attention. The term aromatic is derived from Greek word aroma which means pleasant smelling compounds. But in present form, aromatic compounds are cyclic compounds which contain one or more benzene type rings.
Benzene can undergo substitutions at its ortho, para and meta positions. Therefore, it can form three substituted derivatives.

These are respectively called ortho, meta or para derivatives.
TNT or 2,4,6-Trinitrotoluene contains a tri-substituted benzene ring with a methyl group where the-\[{\text{N}}{{\text{O}}_{\text{2}}}\] groups occupy the two ortho and one para positions. The structure of the compound will be as follows;

Tri and poly substituted derivatives are named by numbering the chain in such a way that the parent group gets the lowest number and sum of the positions of the substituents are the smallest. So, the compound is named as 2,4,6-Trinitrotoluene and has the molecular formula as \[{{\text{C}}_{\text{7}}}{{\text{H}}_{\text{5}}}{{\text{N}}_{\text{3}}}{{\text{O}}_{\text{6}}}\] \[\left( {{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{2}}}\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right){{\left( {{\text{N}}{{\text{O}}_{\text{2}}}} \right)}_{\text{3}}}} \right){\text{.}}\]
This compound is prepared by the continuous nitration toluene, ( \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{C}}{{\text{H}}_{\text{3}}}\]) and then it is crystallized from dilute nitric acid.
Additional Information:
TNT is a solid organic nitrogen compound which is mainly used as an explosive. It was widely used by the militaries of different countries during war time. It can explode under intense heat conditions.
Note: TNT is most favoured as an explosive and is extensively used in demolitions and munitions. It can also be used as an intermediate in the production of dyes and photographic chemicals. It is prepared by treating toluene with nitric acid continuously until the three positions in the benzene ring will be substituted by the- $NO_2$ groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




