
Give the structure and IUPAC name of N,N-Dimethyl-tert-butylamine.
Answer
565.8k+ views
Hint: IUPAC nomenclature stands for the International Union of Pure and Applied Chemistry. IUPAC naming is used in organic chemistry for the naming of organic compounds like iso-hexane. The IUPAC name for iso-hexane is 2-methyl pentane, 3-methyl pentane, 2,3-dimethyl butane.
IUPAC names can easily be derived by the structure of the compound. As the structure of compounds will help to identify the longest chain to name the molecule.
IUPAC rules are used to name organic compounds systematically.
Complete step by step answer:
First, we draw the structure of the compound,
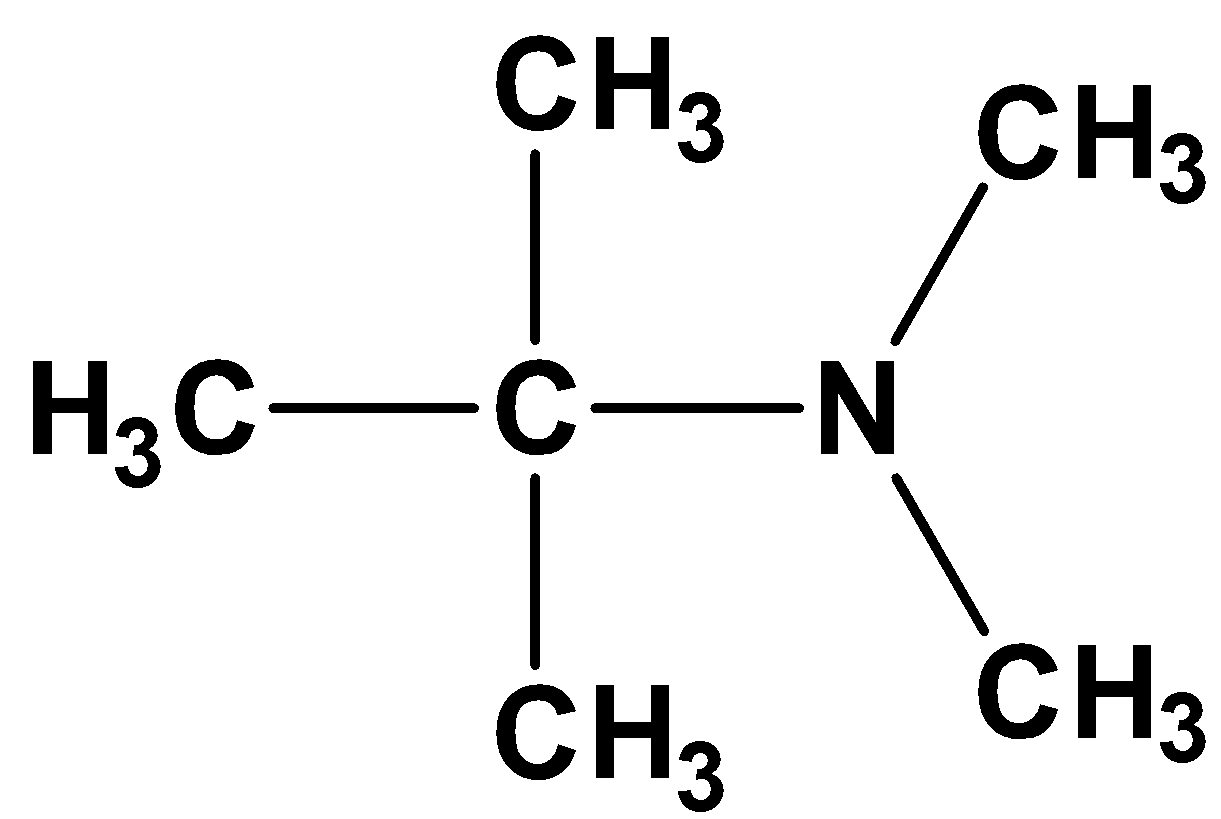
The valency of the nitrogen atom is 3 and the carbon atom next to nitrogen is tetravalent.
Now, we do the IUPAC nomenclature.
The steps followed for IUPAC nomenclature are as follows:–
(1) Naming the longest continuous carbon chain.
Here, the longest continuous carbon chain is propane.
(2) Identifying the functional groups attached to the chain.
In the above example, the functional groups attached to the carbon chain are amine and methyl groups.
(3) Numbering the carbon chain
For starting the numbering, choose the end nearest to a substituent group.
By applying all the rules, we can conclude that the IUPAC name of N, N-Dimethyl-tert-butylamine is
Trimethylpropan-2-amine.
Note: IUPAC nomenclature has drawbacks too. The main drawback is related to the trivial nomenclature system. Here, it can be understood from the example of phenol as phenol is also named as hydroxy benzene and carbolic acid.
While other drugs i.e. the narcotic drugs are addictive and are also banned in many countries.
Also, frequent and high doses of analgesics are harmful to the kidneys as they reduce the blood flow to the kidney.
IUPAC names can easily be derived by the structure of the compound. As the structure of compounds will help to identify the longest chain to name the molecule.
IUPAC rules are used to name organic compounds systematically.
Complete step by step answer:
First, we draw the structure of the compound,

The valency of the nitrogen atom is 3 and the carbon atom next to nitrogen is tetravalent.
Now, we do the IUPAC nomenclature.
The steps followed for IUPAC nomenclature are as follows:–
(1) Naming the longest continuous carbon chain.
Here, the longest continuous carbon chain is propane.
(2) Identifying the functional groups attached to the chain.
In the above example, the functional groups attached to the carbon chain are amine and methyl groups.
(3) Numbering the carbon chain
For starting the numbering, choose the end nearest to a substituent group.
By applying all the rules, we can conclude that the IUPAC name of N, N-Dimethyl-tert-butylamine is
Trimethylpropan-2-amine.
Note: IUPAC nomenclature has drawbacks too. The main drawback is related to the trivial nomenclature system. Here, it can be understood from the example of phenol as phenol is also named as hydroxy benzene and carbolic acid.
While other drugs i.e. the narcotic drugs are addictive and are also banned in many countries.
Also, frequent and high doses of analgesics are harmful to the kidneys as they reduce the blood flow to the kidney.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




