
Give the structural formula of the following:
1-Propanal.
Answer
600.6k+ views
Hint: Aldehydes are the functional group of 1-propanal. It is an organic compound which has an aldehyde (-CHO) functional group to its structure.
Complete step by step answer:
> Aldehyde is a compound which contains -CHO as the functional group. It consists of a carbonyl center, which is a double-bonded carbon to oxygen, with that carbon also bonded to a hydrogen and to an R group, which is any alkyl or side chain.
> Aldehydes are always attached at the start or the end of the structure. It can be added in between the chain but then it has to be on a branched carbon which is not further attached to any group. They can be either unsaturated, that is they contain carbon-carbon double bonds, or can be saturated. An aldehyde ends with a prefix "-al", like methanal, ethanal, propanal, butanal, etc.
The structure of an aldehyde is given below.
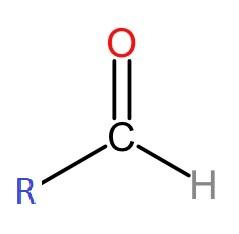
> Now, let us look at the structural formula of 1-propanal. "Prop" means that the compound has three carbon atoms in its structure. The -al as a prefix suggests that it is an aldehyde. And the '1' in its name suggests the position where the aldehyde group is present in the formula, that is on the first carbon. The structural formula is given as:
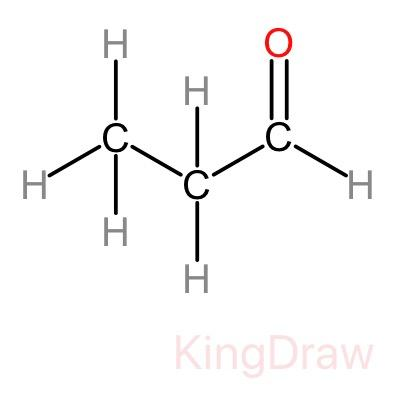
Since propane has a symmetrical structure. Therefore, it does not matter if the aldehyde group is attached at the start of the chain or the end of the chain. The place of the group will remain the same from both sides.
Note: There is a difference between an aldehyde and a ketone. An aldehyde contains -CHO as a functional group. But ketone contains -CO- as a functional group, with a carbon-oxygen double bond. Ketone can only be added in between the chain whereas an aldehyde is added only at the start or end of the chain.
Complete step by step answer:
> Aldehyde is a compound which contains -CHO as the functional group. It consists of a carbonyl center, which is a double-bonded carbon to oxygen, with that carbon also bonded to a hydrogen and to an R group, which is any alkyl or side chain.
> Aldehydes are always attached at the start or the end of the structure. It can be added in between the chain but then it has to be on a branched carbon which is not further attached to any group. They can be either unsaturated, that is they contain carbon-carbon double bonds, or can be saturated. An aldehyde ends with a prefix "-al", like methanal, ethanal, propanal, butanal, etc.
The structure of an aldehyde is given below.

> Now, let us look at the structural formula of 1-propanal. "Prop" means that the compound has three carbon atoms in its structure. The -al as a prefix suggests that it is an aldehyde. And the '1' in its name suggests the position where the aldehyde group is present in the formula, that is on the first carbon. The structural formula is given as:

Since propane has a symmetrical structure. Therefore, it does not matter if the aldehyde group is attached at the start of the chain or the end of the chain. The place of the group will remain the same from both sides.
Note: There is a difference between an aldehyde and a ketone. An aldehyde contains -CHO as a functional group. But ketone contains -CO- as a functional group, with a carbon-oxygen double bond. Ketone can only be added in between the chain whereas an aldehyde is added only at the start or end of the chain.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




