
Give the structural formula of Acetone.
Answer
574.8k+ views
Hint: Acetone is the common name for propanone, it is the smallest ketone existing in nature as the carbonyl group that makes the functional group ketone requires the carbonyl group (>C=O) to be in the middle that is (R-(C=O)-R) or (${R_2} - C = O$).
Complete step by step solution :
Certain rules are set for naming the substituted hydrocarbon chain which are set by IUPAC. Longest hydrocarbon chain is selected as the main chain and other carbons or substitutions are referred to as side chains and less important comparatively.
Other than alkanes, alkenes and alkynes, there are functional groups as well. The functional groups are halides, alcohols, ketones, aldehydes and carboxylic acids, they affect the nomenclature by changing the suffix for alkane hydrocarbon chain.
Since we have to give the structural formula for the above given compound. As the name suggests we figure out the compound contains three carbons and the main functional group in the compound is a ketone as the name in the question is ending with-one. Since acetone is the smallest ketone there should be no confusion as to where the functional group ketone is attached in the compound. Therefore structural formula for the given compound is, $C{H_3}COC{H_3}$
Its structure is given as:
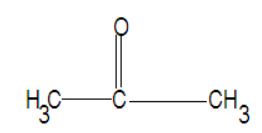
Note:
If there are multiple functional groups in a compound then the functional group with higher seniority changes the suffix while the one with the lower priority changes the prefix. ketone if lower in seniority adds prefix –oxo and if higher, it adds suffix –one
Complete step by step solution :
Certain rules are set for naming the substituted hydrocarbon chain which are set by IUPAC. Longest hydrocarbon chain is selected as the main chain and other carbons or substitutions are referred to as side chains and less important comparatively.
Other than alkanes, alkenes and alkynes, there are functional groups as well. The functional groups are halides, alcohols, ketones, aldehydes and carboxylic acids, they affect the nomenclature by changing the suffix for alkane hydrocarbon chain.
Since we have to give the structural formula for the above given compound. As the name suggests we figure out the compound contains three carbons and the main functional group in the compound is a ketone as the name in the question is ending with-one. Since acetone is the smallest ketone there should be no confusion as to where the functional group ketone is attached in the compound. Therefore structural formula for the given compound is, $C{H_3}COC{H_3}$
Its structure is given as:

Note:
If there are multiple functional groups in a compound then the functional group with higher seniority changes the suffix while the one with the lower priority changes the prefix. ketone if lower in seniority adds prefix –oxo and if higher, it adds suffix –one
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




