
Give the product of the oxidation with jones reagent of $ C{H_3}CH = CHCH{\text{ }}\left( {OH} \right){\text{ }}C{H_3} $ .
Answer
515.1k+ views
Hint: The Jones reagent is suitable for the conversion of secondary alcohols to ketones and most primary alcohols to carboxylic acids. And this is known as jones oxidation. It is a mixture of chromic trioxide in diluted sulfuric acid which is $ Cr{O_3}/{\text{ }}aq{\text{ }}{H_2}S{O_4} $ .
Complete answer:
The molecule that is given above is $ C{H_3}CH = CHCH{\text{ }}\left( {OH} \right){\text{ }}C{H_3} $ . This particular molecule is undergoing oxidation with joins reagent to give the desired product. Jones reagent is $ Cr{O_3}/{\text{ }}aq{\text{ }}{H_2}S{O_4} $ . It converts secondary alcohols to ketones and primary alcohols to carboxylic acids. So we will have to look into our structure to know whether it is secondary alcohol or primary alcohol.
The molecule given in the question can also be written as:
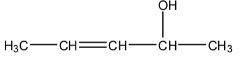
It can be seen above that the molecule is secondary alcohol. Hence the product that is required will contain a ketone group. So let us look at how the reaction of the above molecule will take place and what will be the product of the oxidation reaction.

In the above reaction, the carbon-containing alcohol is secondary so it gets oxidized to a ketone product shown above. There will not be any change in the double-bonded carbons, only the carbon-containing alcohol will change to a ketone.
Therefore the product of the oxidation of $ C{H_3}CH = CHCH{\text{ }}\left( {OH} \right){\text{ }}C{H_3} $ is
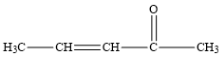
Or $ C{H_3}CH = CHCOC{H_3} $
Note:
if the above compound was primary then the product would contain a carboxylic group in it. Jones reagent can also be prepared by other reagents like sodium dichromate and potassium dichromate. The oxidation reaction with Jones reagent takes place in acetone. It is very rapid and exothermic and its yield is very high.
Complete answer:
The molecule that is given above is $ C{H_3}CH = CHCH{\text{ }}\left( {OH} \right){\text{ }}C{H_3} $ . This particular molecule is undergoing oxidation with joins reagent to give the desired product. Jones reagent is $ Cr{O_3}/{\text{ }}aq{\text{ }}{H_2}S{O_4} $ . It converts secondary alcohols to ketones and primary alcohols to carboxylic acids. So we will have to look into our structure to know whether it is secondary alcohol or primary alcohol.
The molecule given in the question can also be written as:

It can be seen above that the molecule is secondary alcohol. Hence the product that is required will contain a ketone group. So let us look at how the reaction of the above molecule will take place and what will be the product of the oxidation reaction.

In the above reaction, the carbon-containing alcohol is secondary so it gets oxidized to a ketone product shown above. There will not be any change in the double-bonded carbons, only the carbon-containing alcohol will change to a ketone.
Therefore the product of the oxidation of $ C{H_3}CH = CHCH{\text{ }}\left( {OH} \right){\text{ }}C{H_3} $ is

Or $ C{H_3}CH = CHCOC{H_3} $
Note:
if the above compound was primary then the product would contain a carboxylic group in it. Jones reagent can also be prepared by other reagents like sodium dichromate and potassium dichromate. The oxidation reaction with Jones reagent takes place in acetone. It is very rapid and exothermic and its yield is very high.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




