
Give the product of the oxidation of Jones reagent of ${C_6}{H_5}C{H_2}OH$
Answer
527.7k+ views
Hint: Jones reagent is a solution of chromium trioxide $(Cr{O_3})$ in diluted sulfuric acid in a mixture of acetone and water and can be safely used in acetone for oxidation of organic substances. Jones reagent is also suitable for the oxidation of secondary alcohols to ketones and primary alcohols of carboxylic acids and some aldehydes.
Complete answer:
Jones reagent is an oxidizing agent and when primary alcohol reacts with Jones reagent in an acidic media, oxidation reaction takes place and a carboxylic acid is formed because of the hydration of carbonyl group in the ${H_2}O$ solvent.
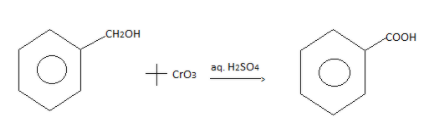
Since the given product is ${C_6}{H_5}C{H_2}OH$ and when it reacts with primary alcohol such as${H_2}S{O_4}$, the product formed will be ${C_6}{H_5}COOH$.
The reaction between the given product and Jones reagent is:
Note:
Jones reagent can also be prepared from sodium dichromate and potassium dichromate. Although the reagent is very acidic, the substrate in acetone is essentially titrated with the oxidant solution and only very acid-sensitive groups are incompatible. Potassium dichromate can be used as an alternative to Chromium dichromate. The reagent rarely oxidizes unsaturated bonds and is very rapid, exothermic and are typically high.
Complete answer:
Jones reagent is an oxidizing agent and when primary alcohol reacts with Jones reagent in an acidic media, oxidation reaction takes place and a carboxylic acid is formed because of the hydration of carbonyl group in the ${H_2}O$ solvent.
Since the given product is ${C_6}{H_5}C{H_2}OH$ and when it reacts with primary alcohol such as${H_2}S{O_4}$, the product formed will be ${C_6}{H_5}COOH$.
The reaction between the given product and Jones reagent is:

Note:
Jones reagent can also be prepared from sodium dichromate and potassium dichromate. Although the reagent is very acidic, the substrate in acetone is essentially titrated with the oxidant solution and only very acid-sensitive groups are incompatible. Potassium dichromate can be used as an alternative to Chromium dichromate. The reagent rarely oxidizes unsaturated bonds and is very rapid, exothermic and are typically high.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




