
Give the IUPAC name of ${\left[ {{\text{Ti}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{3 + }}$. Draw cis and trans isomers of \[[Pt(N{H_3})(C{l_2})]\].
Answer
524.4k+ views
Hint: More complex coordination compounds are made up of an atom or ion (usually a metal) and a ligand-like sequence of bound molecules or anions. The donor atom is the atom in a ligand that is bound to the central atom or ion. Several donor atoms, which may be the same or different, are connected to a standard complex. The coordinate covalent bonds (dipolar bonds) between the ligands and the central atom are referred to as coordination.
Complete answer: The ligands are numbered before the metal ion by naming a complex ion.
In the following sequence, write the names of the ligands: neutral, negative, positive. If several ligands with the same charge class exist, they are called alphabetically. (Numerical prefixes have little impact on the ordering.)
The number of occurrences of several monodentate ligands is indicated by a prefix: di-, tri-, tetra-, penta-, or hexa. Polydentate ligands (e.g., ethylenediamine, oxalate) obtain bis-, tris-, tetrakis-
${\left[ {{\text{Ti}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{3 + }}$=
Metal = Titanium
Ligand = Water = hexaaqua
Charge = +3
IUPAC Name = hexaaquatitanium(III)
Isomerism
"Cis" and "trans" are Latin prefixes that mean "this side of" and "the other side of," respectively. In chemistry, cis denotes functional groups that are on the same side of the carbon chain, while trans denotes functional groups that are on opposite sides of the carbon chain.
The two ligands are on the same side of the complex of cis molecules. Similar ligands are on the opposite sides of the molecules of trans molecules. Stereoisomerism does not exist in tetrahedral molecules. The isomer is called facial, or fac, when three similar ligands share one face.
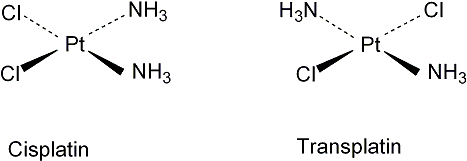
Note:
"Cis" and "trans" are Latin prefixes that mean "this side of" and "the other side of," respectively. In chemistry, cis denotes functional groups that are on the same side of the carbon chain, while trans denotes functional groups that are on opposite sides of the carbon chain.
Complete answer: The ligands are numbered before the metal ion by naming a complex ion.
In the following sequence, write the names of the ligands: neutral, negative, positive. If several ligands with the same charge class exist, they are called alphabetically. (Numerical prefixes have little impact on the ordering.)
The number of occurrences of several monodentate ligands is indicated by a prefix: di-, tri-, tetra-, penta-, or hexa. Polydentate ligands (e.g., ethylenediamine, oxalate) obtain bis-, tris-, tetrakis-
${\left[ {{\text{Ti}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{3 + }}$=
Metal = Titanium
Ligand = Water = hexaaqua
Charge = +3
IUPAC Name = hexaaquatitanium(III)
Isomerism
"Cis" and "trans" are Latin prefixes that mean "this side of" and "the other side of," respectively. In chemistry, cis denotes functional groups that are on the same side of the carbon chain, while trans denotes functional groups that are on opposite sides of the carbon chain.
The two ligands are on the same side of the complex of cis molecules. Similar ligands are on the opposite sides of the molecules of trans molecules. Stereoisomerism does not exist in tetrahedral molecules. The isomer is called facial, or fac, when three similar ligands share one face.
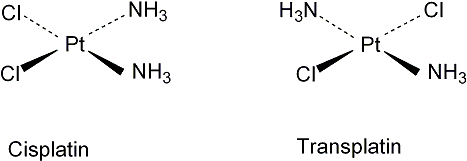
Note:
"Cis" and "trans" are Latin prefixes that mean "this side of" and "the other side of," respectively. In chemistry, cis denotes functional groups that are on the same side of the carbon chain, while trans denotes functional groups that are on opposite sides of the carbon chain.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




