
Give the formula of the following functional groups
(A) Aldehyde
(B) Ketone
Answer
565.5k+ views
Hint: Aldehydes and ketones are organic compounds that have a carbonyl functional group, \[{\text{C = O}}\]. These are compounds with general formula, \[{\text{ - CHO}}\] and , where R and R’ are alkyl groups.
Complete step by step answer:
Aldehydes are compounds where the carbonyl group has one hydrogen atom attached to it along with either a 2nd hydrogen atom or a hydrocarbon group that can be an alkyl group or a group containing a benzene ring.
The smallest aldehyde is formaldehyde.
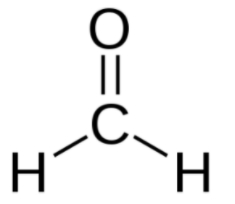
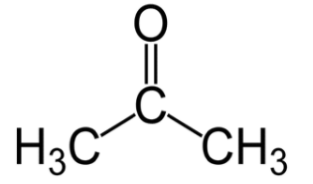
Whereas in ketones, the carbonyl group has two hydrocarbon groups attached to it. The groups can be either containing benzene rings or alkyl groups. A hydrogen atom is not present attached to the carbonyl group in ketone. The smallest ketone is acetone.
Aldehydes and Ketones also known as methanoyl or formyl group. This is because their carbon atom has two remaining bonds that might be bonded to aryl or alkyl or substituents. The compound is a Ketone, If none of these substituents is hydrogen. The compound is an Aldehyde, If at least one of the substituent is hydrogen.
Aldehydes are more reactive than ketones, this is because in ketones, the two alkyl/aryl groups provide steric hindrance during reactions. But in aldehyde, as the hydrogen atom is relatively small, steric hindrance is not present. Also, the electron donating nature of the two alkyl groups reduce the electrophilicity of carbonyl carbon, whereas this effect is less in aldehydes.
Hence, the answer is a formula of functional group in aldehyde is \[{\text{ - CHO}}\] and ketone is $RC\left( { = O} \right)R'$ .
Note: The ketones are more polar than aldehydes.This is due to the presence of two electron-donating R-groups in ketones, this increases the boiling points of ketones and aldehydes.
Aldehydes and ketones have numerous applications due to their chemical properties. Formaldehyde is used in tanning, preparing glues and polymeric products, it is also used as fungicides, germicides and insecticides for plants. It also finds use in and photography drug testing.
Complete step by step answer:
Aldehydes are compounds where the carbonyl group has one hydrogen atom attached to it along with either a 2nd hydrogen atom or a hydrocarbon group that can be an alkyl group or a group containing a benzene ring.
The smallest aldehyde is formaldehyde.

Whereas in ketones, the carbonyl group has two hydrocarbon groups attached to it. The groups can be either containing benzene rings or alkyl groups. A hydrogen atom is not present attached to the carbonyl group in ketone. The smallest ketone is acetone.

Aldehydes and Ketones also known as methanoyl or formyl group. This is because their carbon atom has two remaining bonds that might be bonded to aryl or alkyl or substituents. The compound is a Ketone, If none of these substituents is hydrogen. The compound is an Aldehyde, If at least one of the substituent is hydrogen.
Aldehydes are more reactive than ketones, this is because in ketones, the two alkyl/aryl groups provide steric hindrance during reactions. But in aldehyde, as the hydrogen atom is relatively small, steric hindrance is not present. Also, the electron donating nature of the two alkyl groups reduce the electrophilicity of carbonyl carbon, whereas this effect is less in aldehydes.
Hence, the answer is a formula of functional group in aldehyde is \[{\text{ - CHO}}\] and ketone is $RC\left( { = O} \right)R'$ .
Note: The ketones are more polar than aldehydes.This is due to the presence of two electron-donating R-groups in ketones, this increases the boiling points of ketones and aldehydes.
Aldehydes and ketones have numerous applications due to their chemical properties. Formaldehyde is used in tanning, preparing glues and polymeric products, it is also used as fungicides, germicides and insecticides for plants. It also finds use in and photography drug testing.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




