
Give the formula of magnesium carbonate.
Answer
578.4k+ views
Hint:We know that magnesium carbonate is an inorganic salt that is $MgC{O_3}$ and it is also known as Magnesite or Hydromagnesite or Barringtonite. Hydrated magnesite is present as minerals and they serve as a fertilizer and as an antacid. It’s extensively used in the preparation of materials that are capable of withstanding very elevated temperatures.
Complete step by step answer:
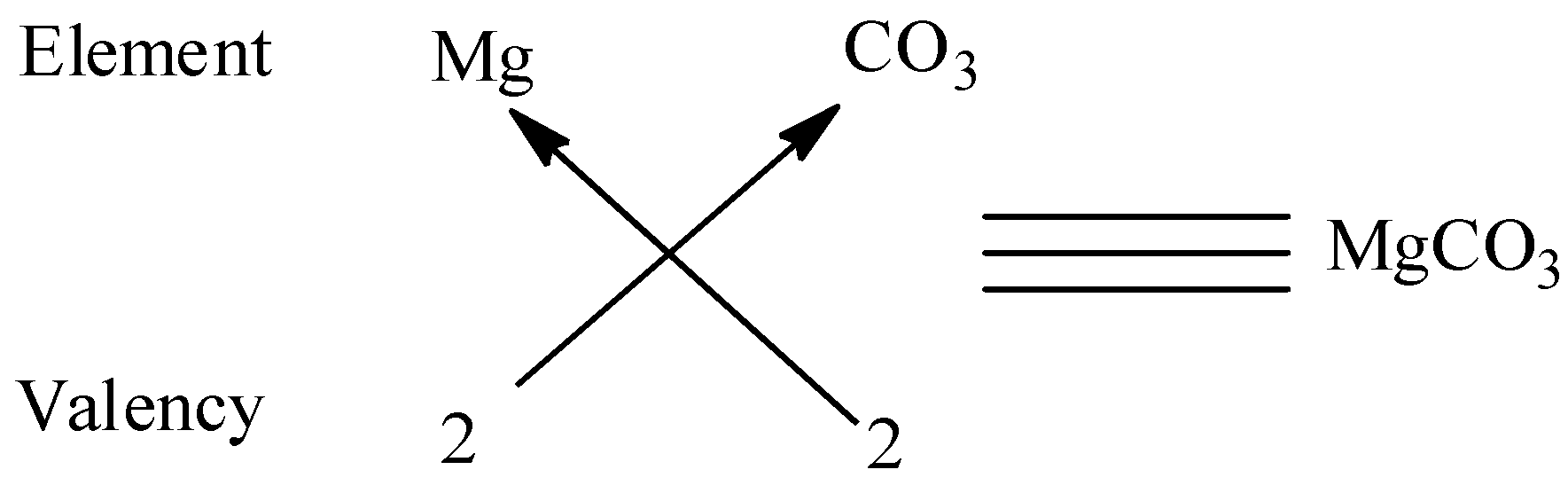
We know that the valency of carbonate \[2\] and the valency of magnesium are \[2\] and to satisfy the combining capacity of carbonate, one magnesium is required. Thus the molecular formula is $MgC{O_3}$.
Additional notes:
We also know that the magnesium carbonate may be a basic hydrated magnesium carbonate or a usually hydrated magnesium carbonate. It presents as a light, white, friable mass or as a bulky white powder. It is neutral and is constant in air. It’s almost insoluble in water to which though it imparts a somewhat alkaline reaction. It is insoluble in alcohol but is dissolved by dilute acids with bubbles. Hydromagnesite may be a white or yellowish or grayish-white or brown colored compound which is produced from crystalline powder or crystalline solid form. It’s a vital ore for magnesium.
Note: Now we discuss about the some of the uses of Magnesium carbonate as:
- Magnesium carbonate is employed in food as a desiccant.
- Utilized in making pharmaceutical products.
- Utilized as a rubber reinforcing agent.
- Utilized in the manufacturing of cosmetics.
- Utilized in making drinking water.
- Utilized as an anti caking agent in food.
- Utilized as a filtering agent.
- Utilized as fire-extinguishing.
- Utilized in printing inks.
Complete step by step answer:
We know that the valency of carbonate \[2\] and the valency of magnesium are \[2\] and to satisfy the combining capacity of carbonate, one magnesium is required. Thus the molecular formula is $MgC{O_3}$.

Additional notes:
We also know that the magnesium carbonate may be a basic hydrated magnesium carbonate or a usually hydrated magnesium carbonate. It presents as a light, white, friable mass or as a bulky white powder. It is neutral and is constant in air. It’s almost insoluble in water to which though it imparts a somewhat alkaline reaction. It is insoluble in alcohol but is dissolved by dilute acids with bubbles. Hydromagnesite may be a white or yellowish or grayish-white or brown colored compound which is produced from crystalline powder or crystalline solid form. It’s a vital ore for magnesium.
Note: Now we discuss about the some of the uses of Magnesium carbonate as:
- Magnesium carbonate is employed in food as a desiccant.
- Utilized in making pharmaceutical products.
- Utilized as a rubber reinforcing agent.
- Utilized in the manufacturing of cosmetics.
- Utilized in making drinking water.
- Utilized as an anti caking agent in food.
- Utilized as a filtering agent.
- Utilized as fire-extinguishing.
- Utilized in printing inks.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




