
Give reasons for the following:
i) Though nitrogen exhibits $ + {\text{5}}$ oxidation state, it does form pentahalide.
ii) Electrons gain enthalpy with negative sign of fluorine is less than that of chlorine.
iii) The two oxygen-oxygen bond lengths in ozone molecules are identical.
Answer
569.4k+ views
Hint: The atomic number of nitrogen is ${\text{7}}$. Find the electronic configuration from the atomic number. The amount of energy released when an electron is added to a gaseous atom is known as the electron gain enthalpy. In ozone, three oxygen atoms are arranged to form a bent shaped structure.
Complete answer:
i) The atomic number of nitrogen is ${\text{7}}$. Thus, the electronic configuration is $1{s^2}\,2{s^2}\,2{p^3}$.
The electronic configuration can be represented as follows:
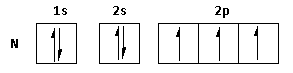
The valence electrons of nitrogen are $2s\,{\text{and }}2p$. Thus, nitrogen loses electrons from these valence orbitals and shows a $ + {\text{5}}$ oxidation state.
But as there are no empty d-orbitals, nitrogen does not form pentahalide. Nitrogen can form trihalide.
ii) The amount of energy released when an electron is added to a gaseous atom is known as the electron gain enthalpy. As the energy is released, the value of electron gain enthalpy has a negative sign. The size of fluorine atom is smaller than that of chlorine atom. As the size of fluorine is smaller, the electronegativity of fluorine atom is greater than that of chlorine atom.
When we try to add an electron to the fluorine atom , due to higher electronegativity, there occurs a repulsion between the electron and fluorine atom. Thus, the force of attraction between the fluorine atom and the electron added is less.
Thus, the electron gain enthalpy of fluorine atom is less negative than that of chlorine atom.
iii) In ozone, there are three oxygen atoms that are arranged to form a bent shaped structure. The central oxygen atom forms a single bond with one terminal oxygen atom and a double bond with the other terminal oxygen atom. But the electrons of the double bond are delocalized over all the three oxygen atoms.
As a result, the single bond and the double bond are the resonance hybrids and not pure. Thus, the oxygen-oxygen bond length is the average bond distance of the single bond and the double bond.

Thus, the two oxygen-oxygen bond lengths in ozone molecules are identical.
Note:The trend in electron gain enthalpy is as follows: Across the period, from left to right, the electron gain enthalpy becomes more and more negative. Down the group, the electron gain enthalpy becomes less negative.
Complete answer:
i) The atomic number of nitrogen is ${\text{7}}$. Thus, the electronic configuration is $1{s^2}\,2{s^2}\,2{p^3}$.
The electronic configuration can be represented as follows:

The valence electrons of nitrogen are $2s\,{\text{and }}2p$. Thus, nitrogen loses electrons from these valence orbitals and shows a $ + {\text{5}}$ oxidation state.
But as there are no empty d-orbitals, nitrogen does not form pentahalide. Nitrogen can form trihalide.
ii) The amount of energy released when an electron is added to a gaseous atom is known as the electron gain enthalpy. As the energy is released, the value of electron gain enthalpy has a negative sign. The size of fluorine atom is smaller than that of chlorine atom. As the size of fluorine is smaller, the electronegativity of fluorine atom is greater than that of chlorine atom.
When we try to add an electron to the fluorine atom , due to higher electronegativity, there occurs a repulsion between the electron and fluorine atom. Thus, the force of attraction between the fluorine atom and the electron added is less.
Thus, the electron gain enthalpy of fluorine atom is less negative than that of chlorine atom.
iii) In ozone, there are three oxygen atoms that are arranged to form a bent shaped structure. The central oxygen atom forms a single bond with one terminal oxygen atom and a double bond with the other terminal oxygen atom. But the electrons of the double bond are delocalized over all the three oxygen atoms.
As a result, the single bond and the double bond are the resonance hybrids and not pure. Thus, the oxygen-oxygen bond length is the average bond distance of the single bond and the double bond.

Thus, the two oxygen-oxygen bond lengths in ozone molecules are identical.
Note:The trend in electron gain enthalpy is as follows: Across the period, from left to right, the electron gain enthalpy becomes more and more negative. Down the group, the electron gain enthalpy becomes less negative.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




