
Give reasons:
A.\[B{F_3}\] has zero dipole moment although the B – F bonds are polar.
B.NaCl is harder than Na metal.
Answer
592.8k+ views
Hint: Dipole moment is a vector quantity. The net dipole moment of a molecule is the vector sum of all the bond dipole moments in the molecule. Also, there is a ionic bond between the articles of sodium and chlorine in sodium chloride (NaCl).
Complete step by step answer:
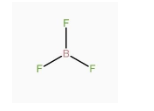
A.\[B{F_3}\] has zero dipole moment although the B – F bonds are polar.
-Dipole moment in any system is due to the separation of charge. It can arise in ionic bonds as well as covalent bonds. It generally occurs due to the difference in electronegativity between the two chemically bonded atoms. Due to the difference in electronegativity, partial positive charge is induced on one atom and partial negative charge is induced on the other atom. The direction of the dipole moment is from the positive charge to the negative charge. -In the given molecule \[B{F_3}\], B – F bonds are polar but the molecule has zero dipole moment because the net dipole moment is the vector sum of all the constituent dipole moments. So, the overall dipole moment will be the vector sum of all the three B – F dipole moments, which are at 120°. The vector sum of any three equal vectors at 120° to each other is equal to zero. Hence, the net dipole moment is zero.
B.NaCl is harder than Na metal
NaCl is an ionic compound formed by the attraction between the positively charged sodium ions and the negatively charged chlorine ions. Thus, there is a strong force of attraction between the constituents which forms a hard crystalline white solid. On the other hand, sodium is metal which involves metallic bonding which is of course weaker than the ionic bonding in NaCl.
Note:
Remember that the dipole moment between any two similar atoms is equal to zero as they both have the same electronegativity. Thus, no charge is induced on either atom.
Complete step by step answer:
A.\[B{F_3}\] has zero dipole moment although the B – F bonds are polar.
-Dipole moment in any system is due to the separation of charge. It can arise in ionic bonds as well as covalent bonds. It generally occurs due to the difference in electronegativity between the two chemically bonded atoms. Due to the difference in electronegativity, partial positive charge is induced on one atom and partial negative charge is induced on the other atom. The direction of the dipole moment is from the positive charge to the negative charge. -In the given molecule \[B{F_3}\], B – F bonds are polar but the molecule has zero dipole moment because the net dipole moment is the vector sum of all the constituent dipole moments. So, the overall dipole moment will be the vector sum of all the three B – F dipole moments, which are at 120°. The vector sum of any three equal vectors at 120° to each other is equal to zero. Hence, the net dipole moment is zero.

B.NaCl is harder than Na metal
NaCl is an ionic compound formed by the attraction between the positively charged sodium ions and the negatively charged chlorine ions. Thus, there is a strong force of attraction between the constituents which forms a hard crystalline white solid. On the other hand, sodium is metal which involves metallic bonding which is of course weaker than the ionic bonding in NaCl.
Note:
Remember that the dipole moment between any two similar atoms is equal to zero as they both have the same electronegativity. Thus, no charge is induced on either atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




