
Give one word or phrase for the following statement. The tendency of an element to form chains of identical atoms.
Answer
541.8k+ views
Hint : The bonding of atoms of the same element into a series is called a chain. The tendency of an element to form chains of identical atoms is mainly formed in carbon because it can form compounds with several other elements. These are capable of forming an expansive range of catenae, including hydrogen, boron, silicon, phosphorus, and sulphur.
Complete Step By Step Answer:
The tendency of an element to form chains of identical atoms. A chain or a ring shape may be open if its ends are not bonded to each other (an open chain compound) or closed if they are bonded in a ring (a cyclic compound)
Catenation occurs most readily with carbon, which forms covalent bonds with other carbon atoms to form covalent bonds with other carbon atoms to form longer chains and structures. This is the reason for the presence of the number of organic compounds in nature, carbon is most well-known for its properties of catenation.
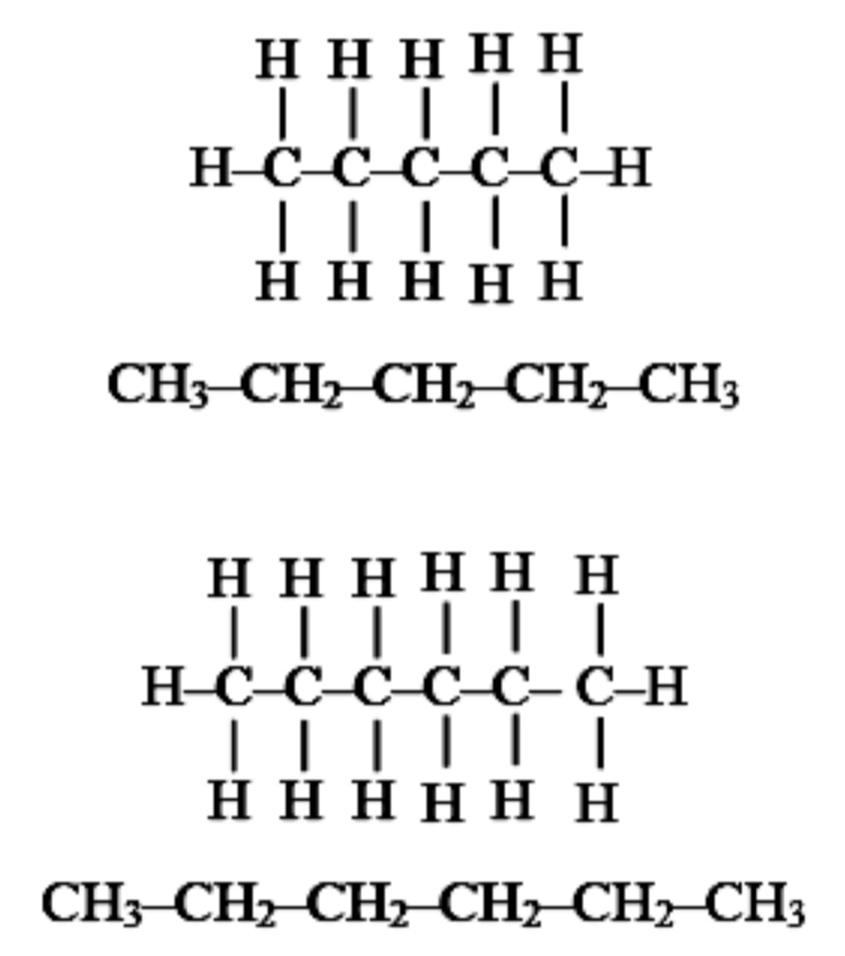
Carbon chains in chemistry can combine with any of various other elements including hydrogen, oxygen, non- metals and metals to form various compounds.
However, carbon is the only element capable of forming various other main groups elements which are capable of forming a large range of chain including hydrogen, oxygen, boron, silicon, phosphorus, nitrogen and sulphur.
Therefore, carbon which has the least diffuse valence shell p orbital is capable of forming p-p sigma bonded chains with atoms than other heavier elements which bond by higher valence shell orbitals.
The molecular orbital and its ability to form different kinds of covalent bonds. For carbon atoms, the sigma overlap between adjacent atoms is strong enough for perfectly stable chains to be formed.
Hence, the tendency of an element to form chains of identical atoms is called Catenation.
Note :
The ability of an element to catenate is basically based on the bond energy of its own element which decreases with more diffuse orbitals because of those with higher azimuthal quantum number overlap to form the various bonds. Catenation ability of an element is also influenced by other factors such as a range of steric and electronic factors. Also it is affected by the electronegativity of the element.
Complete Step By Step Answer:
The tendency of an element to form chains of identical atoms. A chain or a ring shape may be open if its ends are not bonded to each other (an open chain compound) or closed if they are bonded in a ring (a cyclic compound)
Catenation occurs most readily with carbon, which forms covalent bonds with other carbon atoms to form covalent bonds with other carbon atoms to form longer chains and structures. This is the reason for the presence of the number of organic compounds in nature, carbon is most well-known for its properties of catenation.

Carbon chains in chemistry can combine with any of various other elements including hydrogen, oxygen, non- metals and metals to form various compounds.
However, carbon is the only element capable of forming various other main groups elements which are capable of forming a large range of chain including hydrogen, oxygen, boron, silicon, phosphorus, nitrogen and sulphur.
Therefore, carbon which has the least diffuse valence shell p orbital is capable of forming p-p sigma bonded chains with atoms than other heavier elements which bond by higher valence shell orbitals.
The molecular orbital and its ability to form different kinds of covalent bonds. For carbon atoms, the sigma overlap between adjacent atoms is strong enough for perfectly stable chains to be formed.
Hence, the tendency of an element to form chains of identical atoms is called Catenation.
Note :
The ability of an element to catenate is basically based on the bond energy of its own element which decreases with more diffuse orbitals because of those with higher azimuthal quantum number overlap to form the various bonds. Catenation ability of an element is also influenced by other factors such as a range of steric and electronic factors. Also it is affected by the electronegativity of the element.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




