
Give an example of zone refining of metals.
Answer
600.6k+ views
Hint: As the name suggests, ‘zone refining’ is a method for refining or purifying metals. This is also known as zone melting, because purification is done by melting the impurities.
Complete step by step answer:
Let us begin by understanding the term zone refining. It is defined as “a technique for the purification of a crystalline material and especially a metal in which a molten region travels through the material to be refined, picks up impurities at its advancing edge, and then allows the purified part to recrystallize at its opposite edge”.
Zone refining is based on the principle that when an impure metal in a molten state is allowed to cool, only the metal crystallizes while the impurities remain in the molten state (mass) or melt.
It can be represented as –
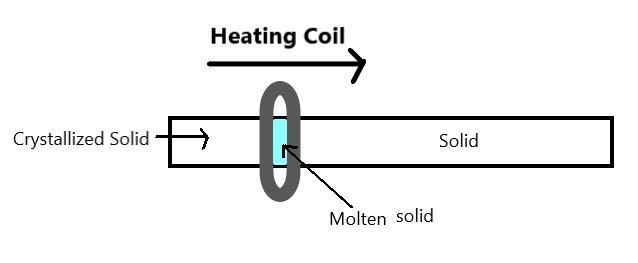
This method is widely used for refining metals that have a low melting point. e.g., tin, lead, bismuth. This method is also very useful for refining metals used as semiconductors, such as – germanium, silicon, boron etc.
Note: The zone refining process is as follows –
A circular mobile heater is fixed at one end of the metal rod (made up of the impure metal)
The circular mobile heater is moved slowly across the impure metal rod
Metallic impurities melt at the temporary position of the heater
The pure metal is left to solidify as the heater moves along the rod
As the heater moves forward, the concentration of the impurities in the melt increases (because solubility of impurity is more)
Impurities are accumulated at one end of the metal rod.
Finally, refined metal is separated from the impurity.
Complete step by step answer:
Let us begin by understanding the term zone refining. It is defined as “a technique for the purification of a crystalline material and especially a metal in which a molten region travels through the material to be refined, picks up impurities at its advancing edge, and then allows the purified part to recrystallize at its opposite edge”.
Zone refining is based on the principle that when an impure metal in a molten state is allowed to cool, only the metal crystallizes while the impurities remain in the molten state (mass) or melt.
It can be represented as –

This method is widely used for refining metals that have a low melting point. e.g., tin, lead, bismuth. This method is also very useful for refining metals used as semiconductors, such as – germanium, silicon, boron etc.
Note: The zone refining process is as follows –
A circular mobile heater is fixed at one end of the metal rod (made up of the impure metal)
The circular mobile heater is moved slowly across the impure metal rod
Metallic impurities melt at the temporary position of the heater
The pure metal is left to solidify as the heater moves along the rod
As the heater moves forward, the concentration of the impurities in the melt increases (because solubility of impurity is more)
Impurities are accumulated at one end of the metal rod.
Finally, refined metal is separated from the impurity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




