
Geometrical shapes of complexes formed by the reaction of ${\text{N}}{{\text{i}}^{{\text{ + 2}}}}$ with ${\text{C}}{{\text{l}}^{\text{ - }}}{\text{,C}}{{\text{N}}^{\text{ - }}}$and ${{\text{H}}_{\text{2}}}{\text{O}}$, respectively are:
(A)Octahedral, tetrahedral and square planar
(B)Tetrahedral, square planar, octahedral
(C)Square planar, tetrahedral, octahedral
(D)Octahedral, square planar, octahedral
Answer
579.6k+ views
Hint: Nickel(II) forms complex compounds with most of the ligands forming tetrahedral, square planar and octahedral geometries. ${\text{N}}{{\text{i}}^{{\text{ + 2}}}}$reacts with ${\text{C}}{{\text{l}}^{\text{ - }}}$to form anionic complex ${\left[ {{\text{NiC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}$, with${\text{C}}{{\text{N}}^{\text{ - }}}$ it forms anionic complex \[{\left[ {{\text{Ni}}{{\left( {{\text{CN}}} \right)}_{\text{4}}}} \right]^{{\text{2 - }}}}\], with ${{\text{H}}_{\text{2}}}{\text{O}}$ it forms ${\left[ {{\text{Ni}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{6}}}} \right]^{{\text{2 + }}}}$ion.
Complete step by step solution:
We now know that Nickel in its +2 oxidation state reacts with chloride ions, cyanide ions and hydroxyl ions of water molecules to form different complexes. Let us get to know the detailed formation of the complexes and their geometries.
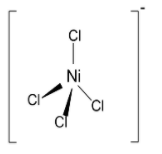
Nickel(II) reacts with chloride ions $\left( {{\text{C}}{{\text{l}}^{\text{ - }}}} \right)$ to from a tetrahedral anionic complex ${\left[ {{\text{NiC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}$ named as tetrachloronickelate (II) ion. The structure of tetrachloronickelate(ii) ions has tetrahedral geometry. The geometry in this reaction changes from octahedral to tetrahedral as the ligand exchange takes place between water and chloride ions. But the oxidation state of nickel does not change from +2. The overall complex charge changes from +2 to -2.
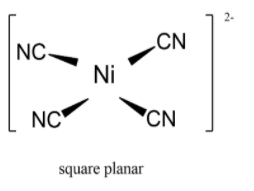
Nickel(II) reacts with cyanide ions to form a square planar complex ion \[{\left[ {{\text{Ni}}{{\left( {{\text{CN}}} \right)}_{\text{4}}}} \right]^{{\text{2 - }}}}\] named as tetracyanonickelate (II) ion. The geometry of tetracyanonickelate (II) ions is square planar geometry. The stoichiometric reaction is ${\left[ {{\text{Ni}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{6}}}} \right]^{{\text{2 + }}}}{\text{ + 4C}}{{\text{N}}^{\text{ - }}} \to {\left[ {{\text{Ni}}{{\left( {{\text{CN}}} \right)}_{\text{4}}}} \right]^{{\text{2 - }}}}{\text{ + 6}}{{\text{H}}_{\text{2}}}{\text{O}}$. In this reaction the geometry of the complex changes from octahedral to square planar.
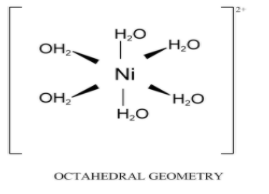
Nickel (II) reacts with six hydroxyl ions from six molecules of water to form an octahedral complex ion called hexaaquanickel(II) ion. The stoichiometric chemical equation is \[{\left[ {{\text{Ni}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{6}}}} \right]^{{\text{2 + }}}}{\text{ + 2O}}{{\text{H}}^{\text{ - }}} \to \left[ {{\text{Ni}}{{\left( {{\text{OH}}} \right)}_{\text{2}}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{4}}}} \right]{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}}\]. The coordination number of the above complex is 6 and the ligands are all unidentate ligands. This is a ligand exchange reaction example of nickel complex.
So the answer for the given question is option(B) tetrahedral, square planar, octahedral.
Note: The six water molecules occupies six corners of the octahedron in but in \[{\left[ {{\text{Ni}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{6}}}} \right]^{{\text{2 + }}}}\] but in other two complexes have different geometries though ligands are four. The reason is the chloride is a weak field ligand whereas cyanide is a strong field that causes different d-orbital electronic distribution.
Complete step by step solution:
We now know that Nickel in its +2 oxidation state reacts with chloride ions, cyanide ions and hydroxyl ions of water molecules to form different complexes. Let us get to know the detailed formation of the complexes and their geometries.

Nickel(II) reacts with chloride ions $\left( {{\text{C}}{{\text{l}}^{\text{ - }}}} \right)$ to from a tetrahedral anionic complex ${\left[ {{\text{NiC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}$ named as tetrachloronickelate (II) ion. The structure of tetrachloronickelate(ii) ions has tetrahedral geometry. The geometry in this reaction changes from octahedral to tetrahedral as the ligand exchange takes place between water and chloride ions. But the oxidation state of nickel does not change from +2. The overall complex charge changes from +2 to -2.

Nickel(II) reacts with cyanide ions to form a square planar complex ion \[{\left[ {{\text{Ni}}{{\left( {{\text{CN}}} \right)}_{\text{4}}}} \right]^{{\text{2 - }}}}\] named as tetracyanonickelate (II) ion. The geometry of tetracyanonickelate (II) ions is square planar geometry. The stoichiometric reaction is ${\left[ {{\text{Ni}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{6}}}} \right]^{{\text{2 + }}}}{\text{ + 4C}}{{\text{N}}^{\text{ - }}} \to {\left[ {{\text{Ni}}{{\left( {{\text{CN}}} \right)}_{\text{4}}}} \right]^{{\text{2 - }}}}{\text{ + 6}}{{\text{H}}_{\text{2}}}{\text{O}}$. In this reaction the geometry of the complex changes from octahedral to square planar.

Nickel (II) reacts with six hydroxyl ions from six molecules of water to form an octahedral complex ion called hexaaquanickel(II) ion. The stoichiometric chemical equation is \[{\left[ {{\text{Ni}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{6}}}} \right]^{{\text{2 + }}}}{\text{ + 2O}}{{\text{H}}^{\text{ - }}} \to \left[ {{\text{Ni}}{{\left( {{\text{OH}}} \right)}_{\text{2}}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{4}}}} \right]{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}}\]. The coordination number of the above complex is 6 and the ligands are all unidentate ligands. This is a ligand exchange reaction example of nickel complex.
So the answer for the given question is option(B) tetrahedral, square planar, octahedral.
Note: The six water molecules occupies six corners of the octahedron in but in \[{\left[ {{\text{Ni}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{6}}}} \right]^{{\text{2 + }}}}\] but in other two complexes have different geometries though ligands are four. The reason is the chloride is a weak field ligand whereas cyanide is a strong field that causes different d-orbital electronic distribution.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




