
Gabriel synthesis is used for the synthesis of:
A.Primary amines
B.Secondary amines
C.Aldehydes
D.Acids
Answer
582.3k+ views
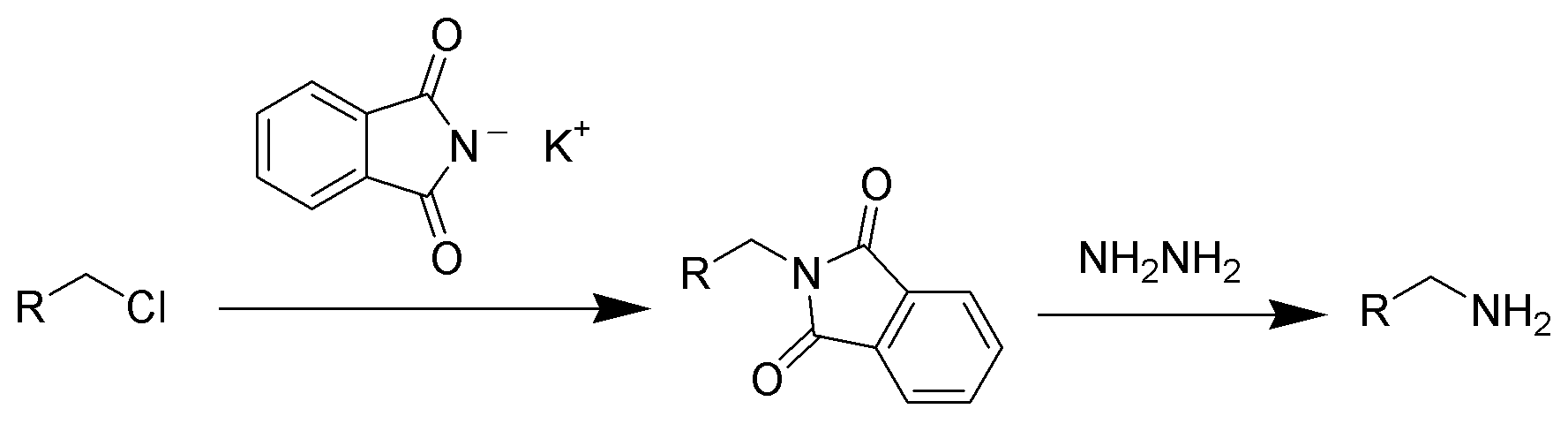
Hint:Gabriel synthesis is a chemical reaction that helps to convert primary alkyl halides into primary amines by using potassium phthalimide. The name of this reaction is named after the German chemist Siegmund Gabriel.
Complete step by step answer:In the Gabriel synthesis method, the sodium or potassium salt of phthalimide is reacted with a primary alkyl halide to form the corresponding N-alkyl phthalimide in the presence of ethanolic potassium hydroxide. After that by the process of acidic hydrolysis, the primary amine is liberated as the amine salt. This reaction includes alkylation of sulfonamides and amide to produce or obtain amines but the alkylation of ammonia is an inefficient and unselective route to obtain amines.
Alcohols are derivatives of water just like these amines are derivatives of ammonia. It has hydrogen with nitrogen and/or alkyl groups attached to it. It has a lone pair of electrons on the nitrogen with the shape of pyramidal around the nitrogen. It can be classified as primary, secondary, and tertiary amines. Primary and secondary amines have H-bond, so it has high boiling points and these are more soluble in water as compared to tertiary amines.
Hence, option A is correct.
Additional information:
Methods for preparation of amines-
SN2 reaction of alkyl halides
The Azide synthesis to produce primary amines
Reduction of nitro compound
Reductive Amination of aldehydes and ketones
Reduction of nitriles and amides
Note: Do not make a mistake in the question where alkyl amines and aromatic amines are present as an option in the reaction of Gabriel synthesis. Because aromatic amines are not produced by the method of Gabriel phthalimide synthesis because aryl halides do not undergo nucleophilic substitution reaction.
Complete step by step answer:In the Gabriel synthesis method, the sodium or potassium salt of phthalimide is reacted with a primary alkyl halide to form the corresponding N-alkyl phthalimide in the presence of ethanolic potassium hydroxide. After that by the process of acidic hydrolysis, the primary amine is liberated as the amine salt. This reaction includes alkylation of sulfonamides and amide to produce or obtain amines but the alkylation of ammonia is an inefficient and unselective route to obtain amines.

Alcohols are derivatives of water just like these amines are derivatives of ammonia. It has hydrogen with nitrogen and/or alkyl groups attached to it. It has a lone pair of electrons on the nitrogen with the shape of pyramidal around the nitrogen. It can be classified as primary, secondary, and tertiary amines. Primary and secondary amines have H-bond, so it has high boiling points and these are more soluble in water as compared to tertiary amines.
Hence, option A is correct.
Additional information:
Methods for preparation of amines-
SN2 reaction of alkyl halides
The Azide synthesis to produce primary amines
Reduction of nitro compound
Reductive Amination of aldehydes and ketones
Reduction of nitriles and amides
Note: Do not make a mistake in the question where alkyl amines and aromatic amines are present as an option in the reaction of Gabriel synthesis. Because aromatic amines are not produced by the method of Gabriel phthalimide synthesis because aryl halides do not undergo nucleophilic substitution reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




