
Froth floatation process of concentration is based on the:
A. preferential wetting properties with frothing agent and water
B. difference in the specific gravities of gangue and ore particles
C. difference in solubility of gangue and ore particles in frothing agent and water
D. difference in reactivity of gangue and ore particles with water and frothing agent
Answer
584.4k+ views
Hint: We must know that froth flotation is a process for selective separation of hydrophobic materials from hydrophilic ones. This process finds application in mineral processing, paper recycling and waste-water treatment industries. Further, the development of froth flotation has helped to improve the recovery of valuable minerals, such as copper- and lead-bearing minerals.
Complete step by step solution:
We can use the froth floatation process mainly for concentration of sulphide ores. In this method, the crushed ore is suspended in water and air is passed using a motor into the suspension. To this mixture, a collector such as pine oil is added so that it selectively wets the ore while water wets the surface of gangue particles. Now, when air is bubbled into the mixture, wet particles act as froth and float at the top being lighter. The froth is then skimmed off and separated from gangue. It is dried and then the metal is obtained.
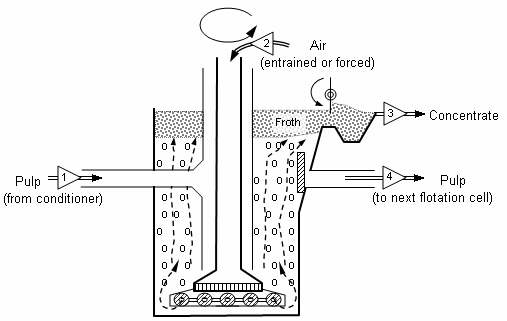
This technique is used in the mining industry and typically separate particles of interest are physically separated from a liquid phase based on the ability of air bubbles to selectively adhere to the surface of the particles, based upon their hydrophobicity.
Therefore, we can conclude that the froth floatation process is based on the preferential wetting properties with the frothing agent and water. Hence, the correct option is C.
Note: We can use this process for many various applications. few examples, we can use it in separating sulfide minerals from silica gangue , separating potassium chloride (sylvite) from sodium chloride (halite),separating coal from ash-forming minerals, removing silicate minerals from iron ores and also separating phosphate minerals from silicates.
Complete step by step solution:
We can use the froth floatation process mainly for concentration of sulphide ores. In this method, the crushed ore is suspended in water and air is passed using a motor into the suspension. To this mixture, a collector such as pine oil is added so that it selectively wets the ore while water wets the surface of gangue particles. Now, when air is bubbled into the mixture, wet particles act as froth and float at the top being lighter. The froth is then skimmed off and separated from gangue. It is dried and then the metal is obtained.

This technique is used in the mining industry and typically separate particles of interest are physically separated from a liquid phase based on the ability of air bubbles to selectively adhere to the surface of the particles, based upon their hydrophobicity.
Therefore, we can conclude that the froth floatation process is based on the preferential wetting properties with the frothing agent and water. Hence, the correct option is C.
Note: We can use this process for many various applications. few examples, we can use it in separating sulfide minerals from silica gangue , separating potassium chloride (sylvite) from sodium chloride (halite),separating coal from ash-forming minerals, removing silicate minerals from iron ores and also separating phosphate minerals from silicates.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




