
From the table given below, answer the questions that follow.
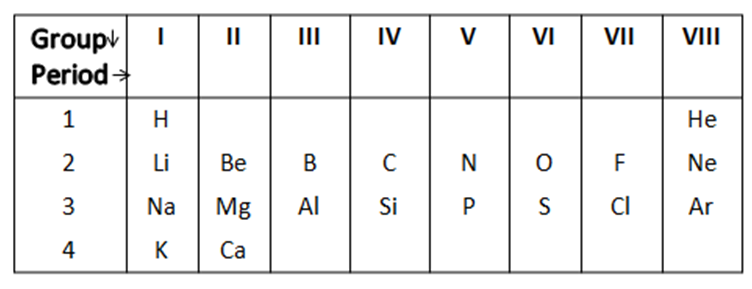
i) Na has physical and chemical properties similar to which element(s) and why?
ii) Write the electronic configuration of N and P. Which one of these will be more electronegative and why?
iii) State a chemical property common to fluorine and chlorine.

Answer
551.7k+ views
Hint:Elements that contain same number of valence electrons will tend to show similar chemical properties because the usually the valance electrons determine the reactivity of the element and hence the chemical properties. When we move down the group, the electronegativity of the elements decreases
Complete step-by-step answer:i) In the table given, we know that the elements from the same group have same valancy and this valancy accounts for the reactive nature of that particular element.
Hence, we can say that sodium which belongs to Group $1$ has similar physical and chemical properties with the elements from Group $1$ i.e. Lithium, Potassium etc.
ii) We know that the atomic number of Nitrogen is $7$ and hence we can write its electronic configuration as $1{s^2}2{s^2}2{p^3}$
Similarly, the electronic configuration of Phosphorous is $15$ and we can write its electronic configuration as $1{s^2}2{s^2}2{p^6}3{s^2}3{p^3}$
Here, we can see that both Nitrogen and Phosphorous belong to the same group and have same number of valance electrons. As, we move down the group the distance between nucleus and the valance electrons increase and therefore decreasing the attraction, this in turn decreases the atoms attractive nature towards electrons or protons.
Therefore, Nitrogen is more electronegative than Phosphorous.
iii) Both Chlorine and Fluorine only accept electrons and do not donate.
Note:In conclusion, Sodium has similar chemical and physical properties with first group elements. Compared to Phosphorous, Nitrogen has more electronegativity. Chlorine and Fluorine only accept electrons and do not donate.
Complete step-by-step answer:i) In the table given, we know that the elements from the same group have same valancy and this valancy accounts for the reactive nature of that particular element.
Hence, we can say that sodium which belongs to Group $1$ has similar physical and chemical properties with the elements from Group $1$ i.e. Lithium, Potassium etc.
ii) We know that the atomic number of Nitrogen is $7$ and hence we can write its electronic configuration as $1{s^2}2{s^2}2{p^3}$
Similarly, the electronic configuration of Phosphorous is $15$ and we can write its electronic configuration as $1{s^2}2{s^2}2{p^6}3{s^2}3{p^3}$
Here, we can see that both Nitrogen and Phosphorous belong to the same group and have same number of valance electrons. As, we move down the group the distance between nucleus and the valance electrons increase and therefore decreasing the attraction, this in turn decreases the atoms attractive nature towards electrons or protons.
Therefore, Nitrogen is more electronegative than Phosphorous.
iii) Both Chlorine and Fluorine only accept electrons and do not donate.
Note:In conclusion, Sodium has similar chemical and physical properties with first group elements. Compared to Phosphorous, Nitrogen has more electronegativity. Chlorine and Fluorine only accept electrons and do not donate.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




