
From the following compounds which compounds have $PC{l_3}$ as starting material?
(A) Organophosphorus compounds
(B) Organonitrogen compounds
(C) Organolithium compounds
(D) Organo compounds
Answer
538.2k+ views
Hint:Here, they have asked about which type of compounds $PC{{l}_{3}}$ can give upon its reaction with different compounds. Phosphorus atoms can form bonds with carbon atoms acting as either nucleophiles or an electrophile.
Complete step-by-step answer:In chemistry, precursor means a compound that can be converted into certain types of chemical compounds. Here, we are asked that $PC{{l}_{3}}$ will give which type of compound as a product when it will react with other compounds.
- $PC{{l}_{3}}$ is called Phosphorus trichloride in which phosphorus is in +3 oxidation state.
Let’s see how $PC{{l}_{3}}$ will react with other organic compounds.
$PC{{l}_{3}}$ as an electrophile:
It can act as an electrophile because the phosphorus atom is electrophilic enough that it can be attacked by the nucleophile. Let’s see some of its examples.
\[PC{{l}_{3}}+3Ph-OH\to P{{(OPh)}_{3}}+3HCl\]
Here, in this reaction, we can see that $P{{(OPh)}_{3}}$ gets formed by reaction of $PC{{l}_{3}}$ with phenol. As $P{{(OPh)}_{3}}$ involves phosphorus atoms along with carbon, oxygen and hydrogen atoms, this compound can be categorized as an organophosphorus compound.
- There is another reaction of $PC{{l}_{3}}$ where it reacts with ethanol to give diethyl phosphite. It is shown below.
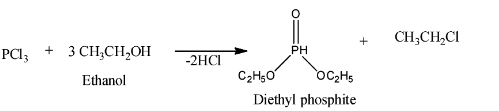
$PC{{l}_{3}}$ as nucleophile:
- $PC{{l}_{3}}$ can also act as a nucleophile as phosphorus atoms has a lone pair of electrons on it. Let’s see its example.
\[PC{{l}_{3}}+R-Cl+AlC{{l}_{3}}\to R-PC{{l}_{3}}^{+}+AlC{{l}_{4}}^{-}\]
Here, we can see that the resulting organic species by the reaction of phosphorus trichloride with alkyl halide and aluminum trichloride has a phosphorus atom as well.
Thus, we can conclude from the above discussion that $PC{{l}_{3}}$ is a precursor to the organometallic compounds.
Therefore option (A) is correct.
Note:As we have seen in the reaction, in most cases, it gives organophosphorus compounds upon its reaction alongside some organic compounds as product as we have seen in its reaction with alcohol. So, do not get confused with that. We can generally say that $PC{{l}_{3}}$ will give an organophosphorus compound upon its reaction with most of the organic compounds.
Complete step-by-step answer:In chemistry, precursor means a compound that can be converted into certain types of chemical compounds. Here, we are asked that $PC{{l}_{3}}$ will give which type of compound as a product when it will react with other compounds.
- $PC{{l}_{3}}$ is called Phosphorus trichloride in which phosphorus is in +3 oxidation state.
Let’s see how $PC{{l}_{3}}$ will react with other organic compounds.
$PC{{l}_{3}}$ as an electrophile:
It can act as an electrophile because the phosphorus atom is electrophilic enough that it can be attacked by the nucleophile. Let’s see some of its examples.
\[PC{{l}_{3}}+3Ph-OH\to P{{(OPh)}_{3}}+3HCl\]
Here, in this reaction, we can see that $P{{(OPh)}_{3}}$ gets formed by reaction of $PC{{l}_{3}}$ with phenol. As $P{{(OPh)}_{3}}$ involves phosphorus atoms along with carbon, oxygen and hydrogen atoms, this compound can be categorized as an organophosphorus compound.
- There is another reaction of $PC{{l}_{3}}$ where it reacts with ethanol to give diethyl phosphite. It is shown below.

$PC{{l}_{3}}$ as nucleophile:
- $PC{{l}_{3}}$ can also act as a nucleophile as phosphorus atoms has a lone pair of electrons on it. Let’s see its example.
\[PC{{l}_{3}}+R-Cl+AlC{{l}_{3}}\to R-PC{{l}_{3}}^{+}+AlC{{l}_{4}}^{-}\]
Here, we can see that the resulting organic species by the reaction of phosphorus trichloride with alkyl halide and aluminum trichloride has a phosphorus atom as well.
Thus, we can conclude from the above discussion that $PC{{l}_{3}}$ is a precursor to the organometallic compounds.
Therefore option (A) is correct.
Note:As we have seen in the reaction, in most cases, it gives organophosphorus compounds upon its reaction alongside some organic compounds as product as we have seen in its reaction with alcohol. So, do not get confused with that. We can generally say that $PC{{l}_{3}}$ will give an organophosphorus compound upon its reaction with most of the organic compounds.
Recently Updated Pages
Master Class 12 Business Studies: Engaging Questions & Answers for Success

Master Class 12 Biology: Engaging Questions & Answers for Success

Master Class 12 Chemistry: Engaging Questions & Answers for Success

Class 12 Question and Answer - Your Ultimate Solutions Guide

Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE




