
What is the formula of oleum?
(a). $ { H }_{ 2 }S{ O }_{ 3 }$
(b). $ { H }_{ 2 }S{ O }_{ 4 }$
(c). ${ H }_{ 2 }S{ O }_{ 5 }$
(d). ${ H }_{ 2 }{ S }_{ 2 }{ O }_{ 7 }$
Answer
613.8k+ views
Hint: Oleum is commonly known as disulphuric acid and oleum is used in the preparation of concentrated sulphuric acid using the contact process by reacting it with water.
Complete step-by-step solution -
Oleum is known by two names: Fuming sulphuric acid and disulphuric acid. As the name suggests, it has two sulphuric acid molecules bound together. So, the formula is ${ H }_{ 2 }{ S }_{ 2 }{ O }_{ 7 }$ which is option d.
Structure of Oleum:
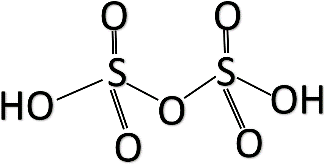
Let us see why the other options are incorrect.
b) The formula of sulphuric acid is ${ H }_{ 2 }S{ O }_{ 4 }$ and it is also known as oil of vitriol. Sulphuric acid is an odourless and colourless liquid and it is highly corrosive.
c) ${ H }_{ 2 }S{ O }_{ 5 }$ is known as Peroxymonosulphuric acid. This acid is also known as Caro’s acid as it has been named after Heinrich Caro who described it for the very first time in the year 1898.
Additional Information: As we know that Oleum is used in the contact process let us know more about the contact process. The contact process is the method of preparing sulphuric acid at the industrial level. Let us see the steps that are involved in the contact process.
• Since excess oxygen is used in the first step, the unused oxygen is passed on for the second step. In this step, the sulphur dioxide formed is converted into sulphur trioxide. This reaction is a reversible reaction so Vanadium pentoxide is used as a catalyst to gain the maximum output in the forward direction. This catalyst favours the forward reaction to the backward reaction.
• In the third step, the sulphur trioxide produced in the last step is dissolved in concentrated sulphuric acid to produce Oleum. As we already know that oleum is known as disulphuric acid, it is further diluted with water to form sulphuric acid as the final product.
Note: Oleum’s formula can sometimes be confused with sulphuric acid since it is also called the fuming sulphuric acid. Oleum is diluted in water to prepare sulphuric acid and oleum as a whole contains two molecules of sulphuric acid joined together.
Complete step-by-step solution -
Oleum is known by two names: Fuming sulphuric acid and disulphuric acid. As the name suggests, it has two sulphuric acid molecules bound together. So, the formula is ${ H }_{ 2 }{ S }_{ 2 }{ O }_{ 7 }$ which is option d.
Structure of Oleum:

Let us see why the other options are incorrect.
- a) $ { H }_{ 2 }S{ O }_{ 3 }$ is known as sulphurous acid and it is often referred to as the aqueous solution of sulphur dioxide ( S${ O }_{ 3 }$ ) . This is because sulphurous acid is prepared by dissolving sulphur dioxide in water.
$S{ O }_{ 2 }+{ H }_{ 2 }O\longrightarrow { H }_{ 2 }{ SO }_{ 3 }$
Additional Information: As we know that Oleum is used in the contact process let us know more about the contact process. The contact process is the method of preparing sulphuric acid at the industrial level. Let us see the steps that are involved in the contact process.
- • In the very first step, sulphur dioxide is prepared by heating sulphur in excess air.
${ S }_{ \left( s \right) }+{ { O }_{ 2 } }_{ \left( g \right) }\longrightarrow S{ { O }_{ 2 } }_{ \left( g \right) }$
${ S }{ { O }_{ 2 } }_{ \left( g \right) }+{ { O }_{ 2 } }_{ \left( g \right) }\overset { { V }_{ 2 }{ O }_{ 5 } }{ \rightleftharpoons } S{ { O }_{ 3 } }_{ \left( g \right) }$
$S{ { O }_{ 3 } }_{ \left( g \right) }+{ H }_{ 2 }{ { SO }_{ 4 } }_{ \left( l \right) }\longrightarrow { H }_{ 2 }{ S }_{ 2 }{ { O }_{ 7 } }_{ \left( l \right) }$
${ H }_{ 2 }{ S }_{ 2 }{ { O }_{ 7 } }_{ \left( l \right) }+{ H }_{ 2 }{ O }_{ \left( l \right) }{ \longrightarrow H }_{ 2 }{ { SO }_{ 4 } }_{ \left( l \right) }$
Note: Oleum’s formula can sometimes be confused with sulphuric acid since it is also called the fuming sulphuric acid. Oleum is diluted in water to prepare sulphuric acid and oleum as a whole contains two molecules of sulphuric acid joined together.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




