
What is the formula of inorganic benzene?
Answer
510.6k+ views
Hint :Inorganic compounds are the compounds that mainly consist of atoms and other carbon. Benzene is a six carbon containing aromatic organic compound that exists as a cyclic structure. The inorganic substitute of benzene must contain six central atoms and a structure similar to benzene.
Complete Step By Step Answer:
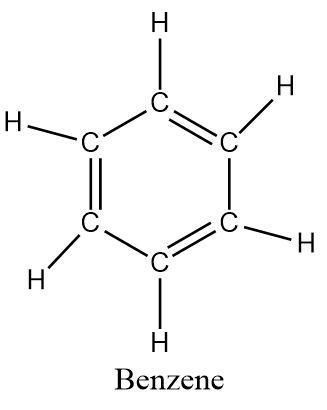
Benzene is an aromatic hydrocarbon with the formula \[{C_6}{H_6}\] .
The structure of the compound is similar to cyclohexane but it has additional double bonds that contribute \[\pi - electrons\] being delocalized in a cyclic manner.
As evident from its formula, benzene contains a total of six carbons atoms attached to each in the form of a regular hexagon with alternative double bonds and a hydrogen attached to each carbon atom.
The inorganic form of benzene is Borazine which contains three boron atoms and three nitrogen atoms along with six hydrogen atoms. Each boron and nitrogen is attached to one hydrogen. The boron atoms and nitrogen atoms are placed alternatively.
Borazine is known as inorganic benzene due its structural similarities with benzene. The boron nitrogen bonds have partial double bond character due to the donation of lone pair of electrons from the nitrogen (which is more electron-rich) to the boron atom (which is electron deficient in nature).
These partial double bonds can alternatively change directions and result in a regular hexagon-like arrangement , similar to that of benzene.
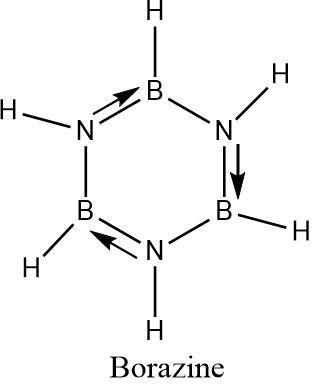
Hence the formula of inorganic benzene (Borazine) is \[{B_3}{N_3}{H_6}\] .
Note :
Borazine is similar to benzene in many ways but also has a lot of differences as all the bonds in benzene are carbon-carbon bonds with no polarity but the bonds in borazine have an electronegativity difference due to which the electrons are more localized on nitrogen and this is reflected in the reactions shown by borazine.
Complete Step By Step Answer:
Benzene is an aromatic hydrocarbon with the formula \[{C_6}{H_6}\] .
The structure of the compound is similar to cyclohexane but it has additional double bonds that contribute \[\pi - electrons\] being delocalized in a cyclic manner.
As evident from its formula, benzene contains a total of six carbons atoms attached to each in the form of a regular hexagon with alternative double bonds and a hydrogen attached to each carbon atom.

The inorganic form of benzene is Borazine which contains three boron atoms and three nitrogen atoms along with six hydrogen atoms. Each boron and nitrogen is attached to one hydrogen. The boron atoms and nitrogen atoms are placed alternatively.
Borazine is known as inorganic benzene due its structural similarities with benzene. The boron nitrogen bonds have partial double bond character due to the donation of lone pair of electrons from the nitrogen (which is more electron-rich) to the boron atom (which is electron deficient in nature).
These partial double bonds can alternatively change directions and result in a regular hexagon-like arrangement , similar to that of benzene.

Hence the formula of inorganic benzene (Borazine) is \[{B_3}{N_3}{H_6}\] .
Note :
Borazine is similar to benzene in many ways but also has a lot of differences as all the bonds in benzene are carbon-carbon bonds with no polarity but the bonds in borazine have an electronegativity difference due to which the electrons are more localized on nitrogen and this is reflected in the reactions shown by borazine.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




