
What is the formula of allyl isocyanide? How many sigma and pi bond are present in it?
Answer
503.4k+ views
Hint: Isocyanide is an organic compound with the functional group $ - N \equiv C $ . It Is an isomer of the related nitrile. Hence the prefix is isocyano. An allyl group is the substituent with the structural formula $ {H_2}C = CH - C{H_2}R $ , where R is the rest of the molecule.
Complete answer:
The formula of allyl isocyanide is $ {C_4}{H_5}N $ .
Sigma bonds $ (\sigma ) $ are the strongest type of covalent bond, formed by head-on overlapping of atomic orbitals.
Pi bonds $ (\pi ) $ are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals.
Every single bond consists of one sigma bond, and that double bond is made up of one sigma and one pi bond. A triple bond consists of one sigma and two pi bonds.
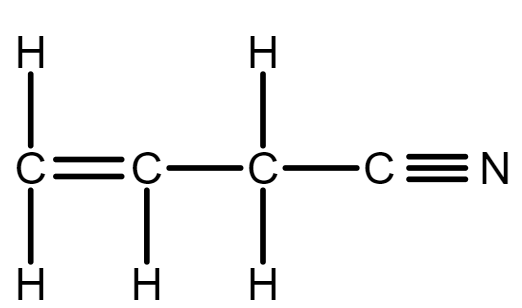
In allyl isocyanide, there is a $ 5 $ single bond between carbon and hydrogen $ C - H $ , and we know in double bond there is one sigma and one pi bond therefore, Three sigma bond between carbon and carbon atoms $ C - C $ . And a triple bond contains one sigma bond and two pi bonds, so there is one sigma bond between carbon and nitrogen atom $ C - N $ .
Total sigma bonds are: $ 5(C - H) + 3(C - C) + 1(C - N) = 9\sigma $ bonds.
One pi bond between $ (C - C) $ and two pi bonds between $ (C - N) $ , therefore, total pi bonds are: $ 1(C - C) + 2(C - N) = 3\pi $ bonds.
So, there are $ 9\sigma $ bonds and $ 3\pi $ bonds in allyl isocyanide.
Note:
There are two non bonded electron pairs in allyl isocyanide, these non bonded electrons are of nitrogen as it has $ 5 $ valence electrons in its outermost shell. It requires $ 3 $ electrons to fulfill its outermost shell, thus Nitrogen makes a triple bond with carbon to get stable.
Complete answer:
The formula of allyl isocyanide is $ {C_4}{H_5}N $ .
Sigma bonds $ (\sigma ) $ are the strongest type of covalent bond, formed by head-on overlapping of atomic orbitals.
Pi bonds $ (\pi ) $ are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals.
Every single bond consists of one sigma bond, and that double bond is made up of one sigma and one pi bond. A triple bond consists of one sigma and two pi bonds.

In allyl isocyanide, there is a $ 5 $ single bond between carbon and hydrogen $ C - H $ , and we know in double bond there is one sigma and one pi bond therefore, Three sigma bond between carbon and carbon atoms $ C - C $ . And a triple bond contains one sigma bond and two pi bonds, so there is one sigma bond between carbon and nitrogen atom $ C - N $ .
Total sigma bonds are: $ 5(C - H) + 3(C - C) + 1(C - N) = 9\sigma $ bonds.
One pi bond between $ (C - C) $ and two pi bonds between $ (C - N) $ , therefore, total pi bonds are: $ 1(C - C) + 2(C - N) = 3\pi $ bonds.
So, there are $ 9\sigma $ bonds and $ 3\pi $ bonds in allyl isocyanide.
Note:
There are two non bonded electron pairs in allyl isocyanide, these non bonded electrons are of nitrogen as it has $ 5 $ valence electrons in its outermost shell. It requires $ 3 $ electrons to fulfill its outermost shell, thus Nitrogen makes a triple bond with carbon to get stable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




