
Formation of (P) is known as Tollens reaction. Identify the total number of stereoisomers for ‘Q’.
\[C{{H}_{3}}C{{H}_{2}}CHO+3HCHCO\xrightarrow[\Delta ]{NaOH}P\xrightarrow[{{H}^{+}}]{C{{H}_{3}}CHO(1eq)}Q\]
Answer
534.6k+ views
Hint: The compounds which are having chiral centers in their structures are going to exhibit stereoisomerism. The formula to calculate the number of stereoisomers in an achiral compound is as follows.
Number of stereoisomers = ${{2}^{n}}$
Here n = number of chiral centers.
Complete answer:
- In the question it is given to identify the product ‘P’ and make the reaction of P with acetaldehyde. We have to find the number of stereoisomers in the compound Q.
- The chemical reaction contains two steps.
Step-1:
- In the question it is given that the formation of P is due to the Tollen’s test means the reactant is an aldehyde. Because aldehydes only responds to Tollen’s test. Alcohols are not going to respond to Tollens test.
- The chemical reaction of propionaldehyde with three moles of formaldehyde is as follows.
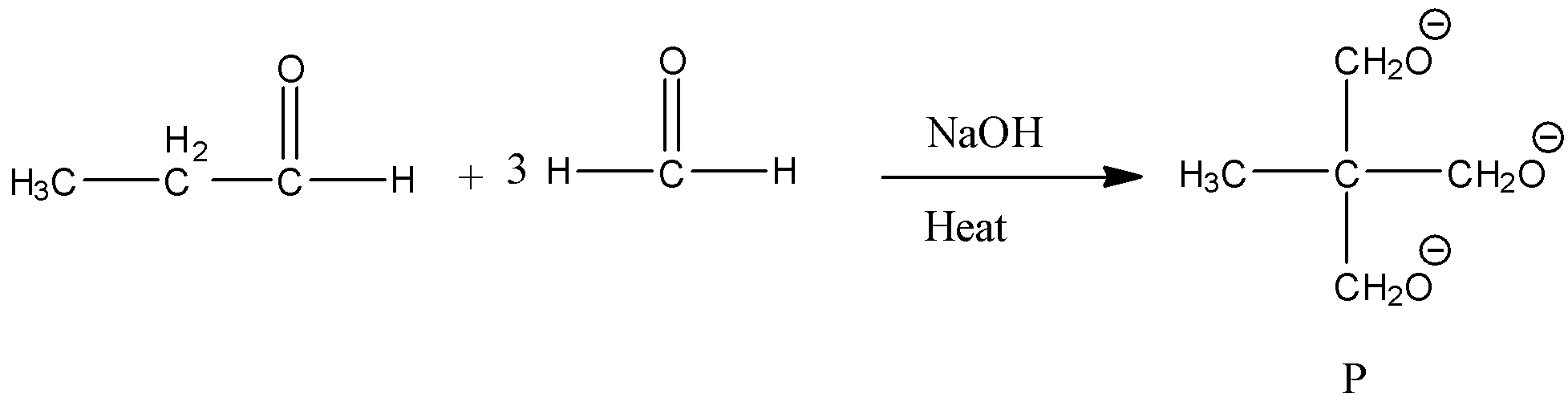
- In the above chemical reaction one mole of propionaldehyde is going to react with three moles of formaldehyde and forms one mole of ‘P’ as the product.
Step-2:
- In the step-2 the product in the above chemical reaction ‘P’ is going to react with one mole of acetaldehyde in the presence of acid and forms the product B and the chemical reaction is as follows.
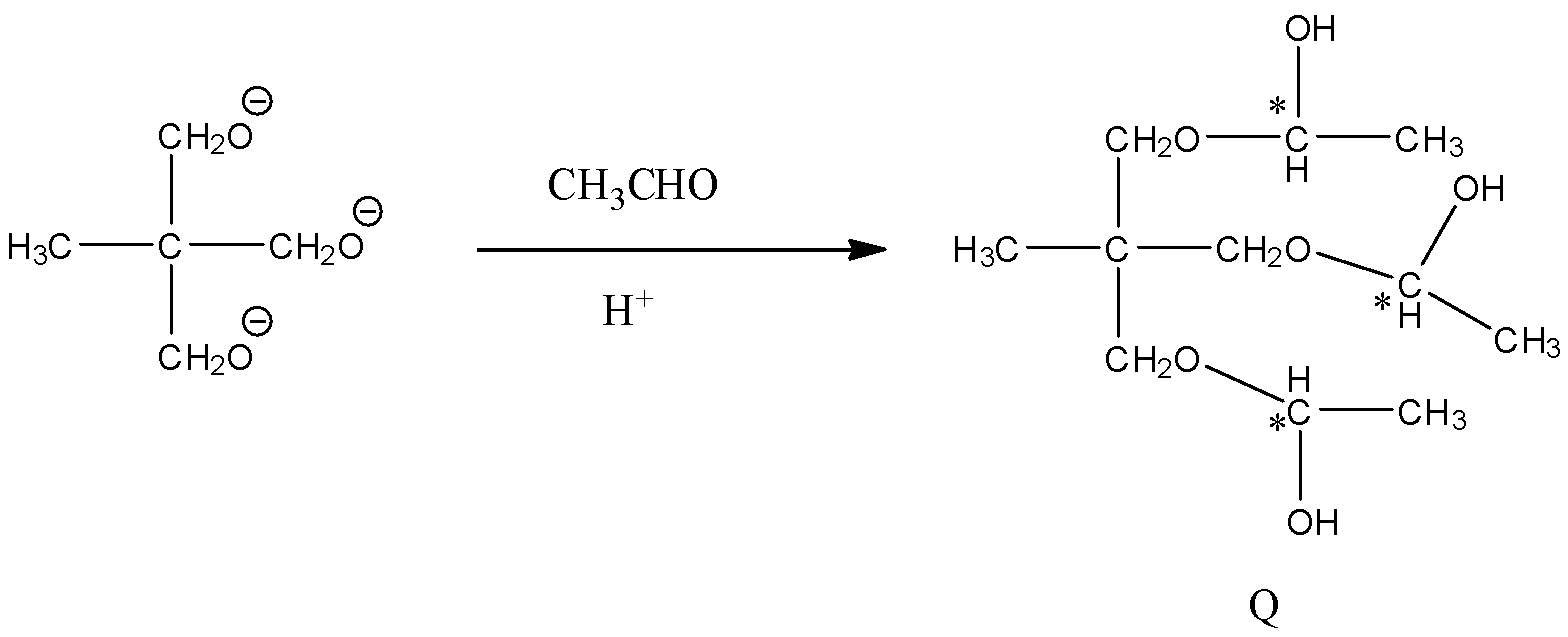
- From the above chemical reaction we can find that the number of chiral centers in the product Q is three and they are represented with star symbols.
- Therefore the number of stereoisomers which are going to be formed by the product B is ${{2}^{3}}=8.$
So, the number of stereoisomers formed by the product B are 8.
Note:
The carbon which contains four different substituents in a molecule then the carbon is called chiral center. Chiral carbon containing molecules are optically active in nature means they rotate the polarized light to either the right or left side.
Number of stereoisomers = ${{2}^{n}}$
Here n = number of chiral centers.
Complete answer:
- In the question it is given to identify the product ‘P’ and make the reaction of P with acetaldehyde. We have to find the number of stereoisomers in the compound Q.
- The chemical reaction contains two steps.
Step-1:
- In the question it is given that the formation of P is due to the Tollen’s test means the reactant is an aldehyde. Because aldehydes only responds to Tollen’s test. Alcohols are not going to respond to Tollens test.
- The chemical reaction of propionaldehyde with three moles of formaldehyde is as follows.

- In the above chemical reaction one mole of propionaldehyde is going to react with three moles of formaldehyde and forms one mole of ‘P’ as the product.
Step-2:
- In the step-2 the product in the above chemical reaction ‘P’ is going to react with one mole of acetaldehyde in the presence of acid and forms the product B and the chemical reaction is as follows.

- From the above chemical reaction we can find that the number of chiral centers in the product Q is three and they are represented with star symbols.
- Therefore the number of stereoisomers which are going to be formed by the product B is ${{2}^{3}}=8.$
So, the number of stereoisomers formed by the product B are 8.
Note:
The carbon which contains four different substituents in a molecule then the carbon is called chiral center. Chiral carbon containing molecules are optically active in nature means they rotate the polarized light to either the right or left side.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




