
Formation of oxonium salts shows that ethers are:
A.Basic in nature
B.Acidic in nature
C.Neutral in nature
D.Amphoteric in nature
Answer
580.2k+ views
Hint: First of all we will consider what are oxonium salts and the reaction involved in their formation. For the preliminary knowledge oxonium salts are formed when ether is reacted with a strong acid.
Complete step by step answer:
Oxonium salts are formed when ethers react with mineral acids such as hydrochloric acid or sulphuric acid. In ethers due to the presence of lone pairs of electrons on oxygen atoms, it behaves as a Lewis base and reacts with a strong acid to form protonated oxonium salt.
As oxygen atoms donate the lone pair of electrons therefore, the ether can be said to be basic in nature.
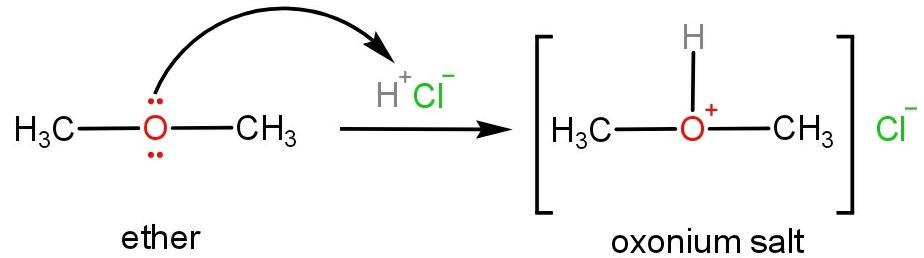
Molecular, atomic or ionic species which have a tendency to donate lone pairs of electrons or accept protons are said to be basic in nature. While species which tend to accept lone pairs of electrons or donate protons are said to be acidic in nature.
Some species act as both acid and base under different conditions. Such species which have both acidic and basic nature are called amphoteric.
Therefore, the answer is option A.
Note:
Students should not misinterpret that the reaction with a strong acid will lead to dehydration in the compound and form a dehydrated product. They should emphasize on the fact that due to the lone pair of electrons on oxygen atoms, it will tend to attract protons dissociated by the strong acid and form salt.
Complete step by step answer:
Oxonium salts are formed when ethers react with mineral acids such as hydrochloric acid or sulphuric acid. In ethers due to the presence of lone pairs of electrons on oxygen atoms, it behaves as a Lewis base and reacts with a strong acid to form protonated oxonium salt.
As oxygen atoms donate the lone pair of electrons therefore, the ether can be said to be basic in nature.

Molecular, atomic or ionic species which have a tendency to donate lone pairs of electrons or accept protons are said to be basic in nature. While species which tend to accept lone pairs of electrons or donate protons are said to be acidic in nature.
Some species act as both acid and base under different conditions. Such species which have both acidic and basic nature are called amphoteric.
Therefore, the answer is option A.
Note:
Students should not misinterpret that the reaction with a strong acid will lead to dehydration in the compound and form a dehydrated product. They should emphasize on the fact that due to the lone pair of electrons on oxygen atoms, it will tend to attract protons dissociated by the strong acid and form salt.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE

Explain sex determination in humans with line diag class 12 biology CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE

What is myopia and hypermetropia How are they corrected class 12 physics CBSE




