
What is the formal charge on the carbon atom in the following two structures?
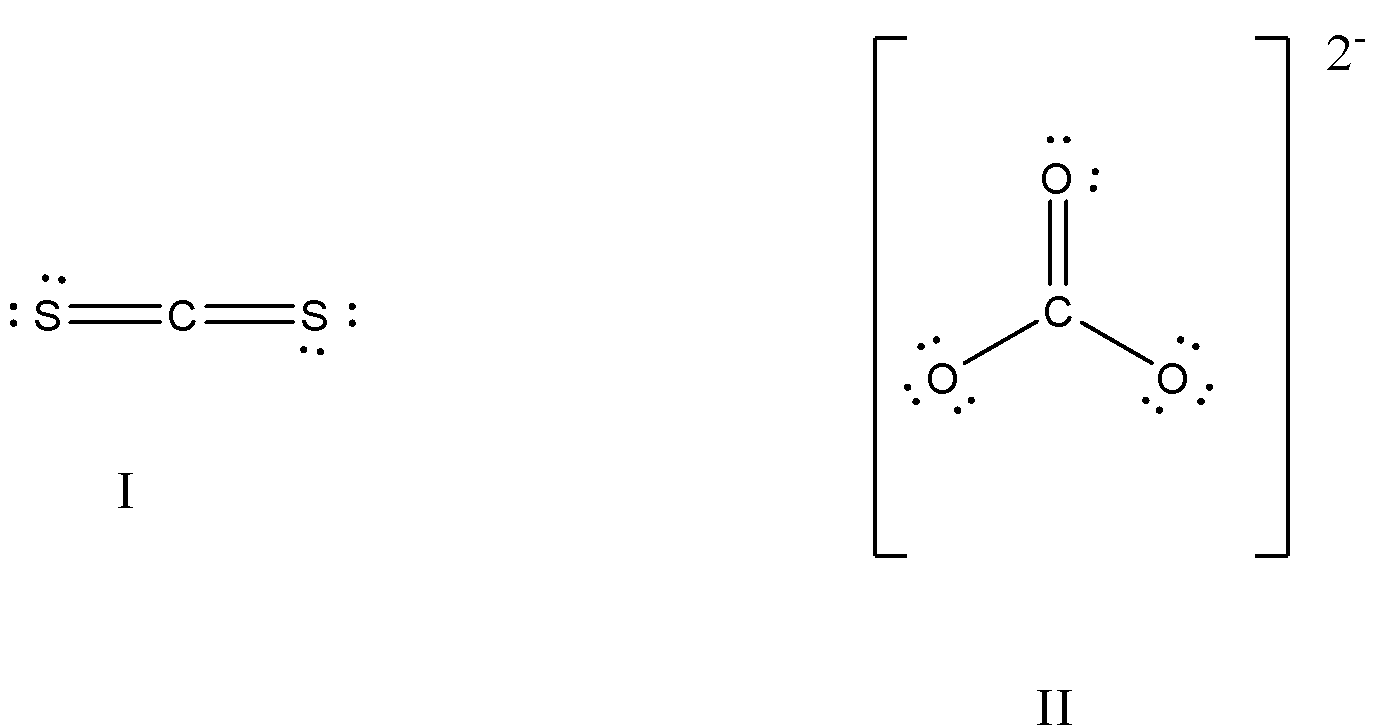
(a)- 0, -2
(b)- 0, 0
(c)- +2, -2
(d)- +1, -1

Answer
584.4k+ views
Hint: The formal charge of an atom in the molecule can be calculated from the total number of valence electrons in the free atom, the total number of electrons of lone pairs i.e., non-bonding electrons, and total number of shared electrons, i.e., bonding electrons.
Complete step by step answer:
The formal charge of an atom in the molecule can be defined as the difference in the number of electrons in the free atom and the number of electrons assigned in the Lewis structure.
The formal charge of an atom in the molecule can be calculated from the total number of valence electrons in the free atom, a total number of electrons of lone pairs i.e., non-bonding electrons, and a total number of shared electrons, i.e., bonding electrons.
The formula is:
$FC=\left[ \begin{align}
& \{\text{Total no}\text{. of valence electrons of atoms in free state }\!\!\}\!\!\text{ - }\!\!\{\!\!\text{ Total no}\text{. of electrons of lone pairs }\!\!\}\!\!\text{ } \\
& \text{ -}\dfrac{1}{2}\text{ }\!\!\{\!\!\text{ Total no}\text{. of shared electrons }\!\!\}\!\!\text{ } \\
\end{align} \right]$ $FC=V-L-\dfrac{1}{2}S$
So, in $C{{S}_{2}}$, the formal charge on carbon will be,
The total number of valence electrons of carbon is 4, there are no lone pairs on carbon and number of bond electrons are 8, so putting all these in the equation, we get
$FC=V-L-\dfrac{1}{2}S=4-0-\dfrac{1}{2}\text{ x 8= 4 - 4 = 0}$
So, the formal charge is 0.
In $CO_{3}^{2-}$, the formal charge of carbon will be,
The total number of valence electrons of carbon is 4, there are no lone pairs on carbon, and the number of bond electrons is 8, so putting all these in the equation, we get
$FC=V-L-\dfrac{1}{2}S=4-0-\dfrac{1}{2}\text{ x 8= 4 - 4 = 0}$
So, the formal charge is 0.
So, the correct answer is “Option B”.
Note: When you are counting the number of shared electrons and don't count the number bonds, you have to calculate the number of electrons involved in the bonds. If the atom has one lone pair then the number of electrons in lone pair will be 2.
Complete step by step answer:
The formal charge of an atom in the molecule can be defined as the difference in the number of electrons in the free atom and the number of electrons assigned in the Lewis structure.
The formal charge of an atom in the molecule can be calculated from the total number of valence electrons in the free atom, a total number of electrons of lone pairs i.e., non-bonding electrons, and a total number of shared electrons, i.e., bonding electrons.
The formula is:
$FC=\left[ \begin{align}
& \{\text{Total no}\text{. of valence electrons of atoms in free state }\!\!\}\!\!\text{ - }\!\!\{\!\!\text{ Total no}\text{. of electrons of lone pairs }\!\!\}\!\!\text{ } \\
& \text{ -}\dfrac{1}{2}\text{ }\!\!\{\!\!\text{ Total no}\text{. of shared electrons }\!\!\}\!\!\text{ } \\
\end{align} \right]$ $FC=V-L-\dfrac{1}{2}S$
So, in $C{{S}_{2}}$, the formal charge on carbon will be,
The total number of valence electrons of carbon is 4, there are no lone pairs on carbon and number of bond electrons are 8, so putting all these in the equation, we get
$FC=V-L-\dfrac{1}{2}S=4-0-\dfrac{1}{2}\text{ x 8= 4 - 4 = 0}$
So, the formal charge is 0.
In $CO_{3}^{2-}$, the formal charge of carbon will be,
The total number of valence electrons of carbon is 4, there are no lone pairs on carbon, and the number of bond electrons is 8, so putting all these in the equation, we get
$FC=V-L-\dfrac{1}{2}S=4-0-\dfrac{1}{2}\text{ x 8= 4 - 4 = 0}$
So, the formal charge is 0.
So, the correct answer is “Option B”.
Note: When you are counting the number of shared electrons and don't count the number bonds, you have to calculate the number of electrons involved in the bonds. If the atom has one lone pair then the number of electrons in lone pair will be 2.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




