
For which of the given molecule dipole moments is not zero,\[\mu \ne 0\]?
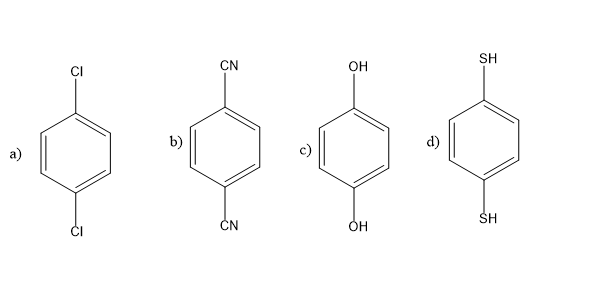

Answer
523.2k+ views
Hint: Dipole moment of the molecule defines the polarity of the bond between two atoms in a molecule. And this polarity between the bonds arises due to the development of the negative or positive charges on the atom sharing the bond.
Complete answer:
Now, we have the basic idea regarding the dipole moment and how it arises, so let’s look at each option.
One more point to keep in mind is that the direction of the dipole is from electronegative atom to the electropositive atom. Dipole moment is designated by the letter\[\mu \].
So looking at the figure \[(c)and(d)\] , quinoa and thioquinol molecules respectively. So the \[ - OH\]group of the quinol molecule has different orientation as shown in figure, thus, it does not cancel out its dipole moment. So, its dipole moment\[\mu \ne 0\].
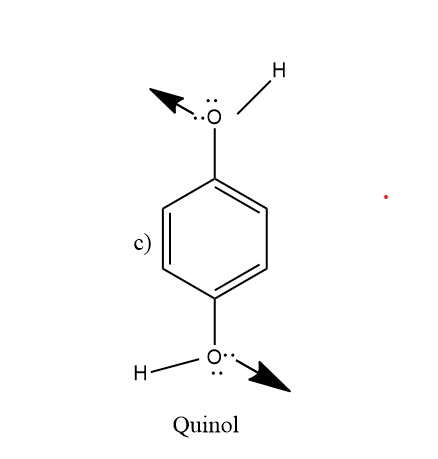
Similarly \[ - SH\] group of thioquinol exists in different conformation and thus does not cancel out their dipole moment. So, its dipole moment is also not zero,\[\mu \ne 0\].
Whereas in case of figure \[(a)and(b)\] , chlorine being the electronegative atoms, at the both ends cancel out the dipole moment and thus its dipole moment is zero \[\mu = 0\]
Same is the case with cyanide groups, due to better symmetry it cancels out its dipole moment. \[\mu = 0\].
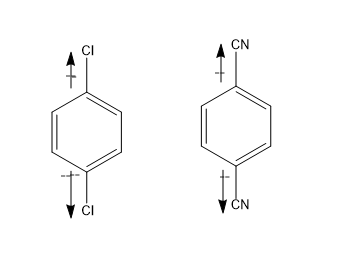
So, option \[(b)\]is the correct answer i.e. figure \[(c)and(d)\] .
Note:
While attempting this type of question, make sure to read the question very carefully, because they are asking for the figure which “do not “show the dipole moment zero. So keep in mind the word not because usually in a hurry we neglect.
Complete answer:
Now, we have the basic idea regarding the dipole moment and how it arises, so let’s look at each option.
One more point to keep in mind is that the direction of the dipole is from electronegative atom to the electropositive atom. Dipole moment is designated by the letter\[\mu \].
So looking at the figure \[(c)and(d)\] , quinoa and thioquinol molecules respectively. So the \[ - OH\]group of the quinol molecule has different orientation as shown in figure, thus, it does not cancel out its dipole moment. So, its dipole moment\[\mu \ne 0\].

Similarly \[ - SH\] group of thioquinol exists in different conformation and thus does not cancel out their dipole moment. So, its dipole moment is also not zero,\[\mu \ne 0\].
Whereas in case of figure \[(a)and(b)\] , chlorine being the electronegative atoms, at the both ends cancel out the dipole moment and thus its dipole moment is zero \[\mu = 0\]
Same is the case with cyanide groups, due to better symmetry it cancels out its dipole moment. \[\mu = 0\].

So, option \[(b)\]is the correct answer i.e. figure \[(c)and(d)\] .
Note:
While attempting this type of question, make sure to read the question very carefully, because they are asking for the figure which “do not “show the dipole moment zero. So keep in mind the word not because usually in a hurry we neglect.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




