
For the given three step endothermic reaction, which of the following statement(s) is/are correct:
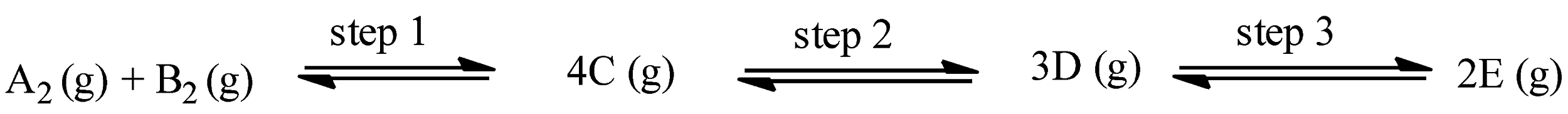
(a)- Step 1 is favored by high temperature and low pressure
(b)- Step 2 is favored by low temperature and high pressure
(c)- Step 2 is favored by high pressure and high temperature
(d)- Step 3 is favored by high pressure and high temperature
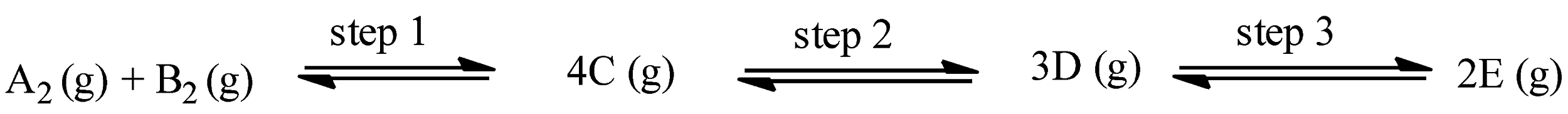
Answer
526.8k+ views
Hint: With the help of endothermic or exothermic property we can calculate the temperature required for the reaction, and for calculating the pressure of the reaction, a change in the number of moles can be seen. Both the factors are calculated according to Le Chatelier’s principle.
Complete answer:
We can solve this question according to Le Chatelier’s principle. So, we have to tell the conditions of temperature are the pressure that must be applied to the reaction, so that forward reaction is preferred.
The given reaction is:
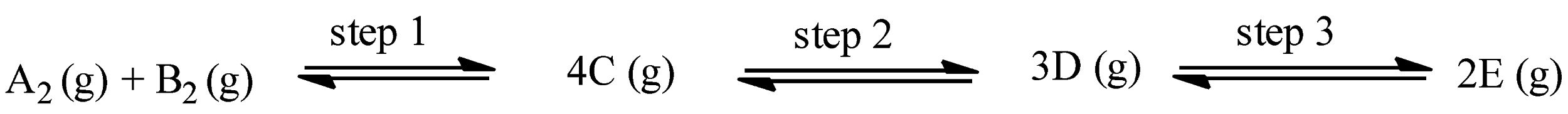
With the help of endothermic or exothermic property, we can calculate the temperature required for the reaction, and for calculating the pressure of the reaction, a change in the number of moles can be seen.
As it is stated that all the steps in the reaction are endothermic reactions, therefore, all the steps will move forward at high temperatures.
In step 1, the number of moles on the product side is 4 and the number of moles on the reactant side is 2, therefore,
$\Delta n=4-2=2$
So, $\Delta n>1$, this step will occur at low pressure.
In step 2, the number of moles on the product side is 3 and the number of moles on the reactant side is 4, therefore,
$\Delta n=3-4=-1$
So, $\Delta n<1$, this step will occur at high pressure.
In step 3, the number of moles on the product side is 2 and the number of moles on the reactant side is 3, therefore,
$\Delta n=2-3=-1$
So, $\Delta n<1$, this step will occur at high pressure.
So, corresponding to the above discussion, options (a), (c), and (d) are correct answers.
Note:
If the reaction given is exothermic then the low temperature is favored for the forward reaction, when the reaction given is endothermic the high temperature is favored for the forward reaction.
Complete answer:
We can solve this question according to Le Chatelier’s principle. So, we have to tell the conditions of temperature are the pressure that must be applied to the reaction, so that forward reaction is preferred.
The given reaction is:
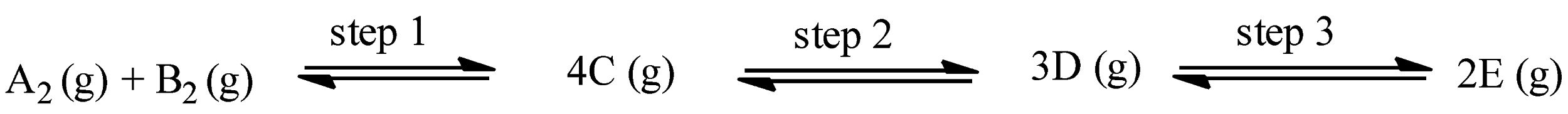
With the help of endothermic or exothermic property, we can calculate the temperature required for the reaction, and for calculating the pressure of the reaction, a change in the number of moles can be seen.
As it is stated that all the steps in the reaction are endothermic reactions, therefore, all the steps will move forward at high temperatures.
In step 1, the number of moles on the product side is 4 and the number of moles on the reactant side is 2, therefore,
$\Delta n=4-2=2$
So, $\Delta n>1$, this step will occur at low pressure.
In step 2, the number of moles on the product side is 3 and the number of moles on the reactant side is 4, therefore,
$\Delta n=3-4=-1$
So, $\Delta n<1$, this step will occur at high pressure.
In step 3, the number of moles on the product side is 2 and the number of moles on the reactant side is 3, therefore,
$\Delta n=2-3=-1$
So, $\Delta n<1$, this step will occur at high pressure.
So, corresponding to the above discussion, options (a), (c), and (d) are correct answers.
Note:
If the reaction given is exothermic then the low temperature is favored for the forward reaction, when the reaction given is endothermic the high temperature is favored for the forward reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




