
For the given reaction $N{{a}_{2}}{{B}_{4}}{{O}_{7}}.10{{H}_{2}}O\overset{\Delta }{\mathop{\to }}\,X\overset{\Delta }{\mathop{\to }}\,Y+Z$
X, Y and Z in the reaction are:
A. \[X=\text{N}{{\text{a}}_{2}}{{\text{B}}_{4}}{{\text{O}}_{7}}\text{, }\!\!~\!\!\text{ } Y=\text{NaB}{{\text{O}}_{2}}, Z={{\text{B}}_{2}}{{\text{O}}_{3}}\]
B. \[X=\text{N}{{\text{a}}_{2}}{{\text{B}}_{4}}{{\text{O}}_{7}}\text{, }\!\!~\!\!\text{ } Y={{\text{B}}_{2}}{{\text{O}}_{3}}, Z={{H}_{3}}B{{O}_{3}}\]
C. \[X=\text{N}{{\text{a}}_{2}}{{\text{B}}_{4}}{{\text{O}}_{7}}~, Y=\text{ }\!\!~\!\!\text{ NaB}{{\text{O}}_{2}}, Z=\text{B}{{\left( \text{OH} \right)}_{3}}\]
D. \[X=\text{N}{{\text{a}}_{2}}{{\text{B}}_{4}}{{\text{O}}_{7}}, Y=\text{ }\!\!~\!\!\text{ }{{\text{B}}_{2}}{{\text{O}}_{3}}, Z=\text{ }\!\!~\!\!\text{ B}{{\left( \text{OH} \right)}_{3}}\]
Answer
597.6k+ views
Hint: When a compound possesses water of crystallisation on heating it first loses the water of crystallisation and on further heating it dissociates into its constituents. Same it with the given compound borax.
Complete answer:
When heated, borax undergoes various transitions which are mentioned as follows: It first loses 10 water molecules which is water of crystallisation and swells up. Then, it turns into a transparent liquid, solidifying to form a glass-like material which is called borax bead. If borax, $N{{a}_{2}}{{B}_{4}}{{O}_{7}}.10{{H}_{2}}O$ is heated more on the platinum loop, then, a transparent colourless glass like bead of sodium metaborate \[(NaB{{O}_{3}})\] and boric anhydride \[({{\text{B}}_{2}}{{\text{O}}_{3}})\] is formed.
$N{{a}_{2}}{{B}_{4}}{{O}_{7}}.10{{H}_{2}}O\overset{\Delta }{\mathop{\to }}\,N{{a}_{2}}{{B}_{4}}{{O}_{7}}\overset{\Delta }{\mathop{\to }}\,2NaB{{O}_{2}}+{{B}_{2}}{{O}_{3}}$
So, answer is option A which is \[X=\text{N}{{\text{a}}_{2}}{{\text{B}}_{4}}{{\text{O}}_{7}}\text{, }\!\!~\!\!\text{ }Y=\text{NaB}{{\text{O}}_{2}},Z={{\text{B}}_{2}}{{\text{O}}_{3}}\]
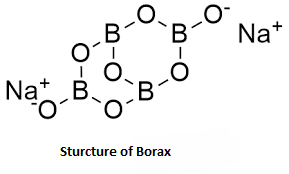
Note: Borax, also called sodium tetraborate, is a powdery white mineral that has been used as a cleaning product for several decades. It has many uses: It helps get rid of stains, mould, and mildew around the house. It can kill insects such as ants. It is an important boron compound, a mineral, and a salt of boric acid. Powdered borax is white, consisting of soft colourless crystals that dissolve easily in water. Borax has a wide variety of uses.
Complete answer:
When heated, borax undergoes various transitions which are mentioned as follows: It first loses 10 water molecules which is water of crystallisation and swells up. Then, it turns into a transparent liquid, solidifying to form a glass-like material which is called borax bead. If borax, $N{{a}_{2}}{{B}_{4}}{{O}_{7}}.10{{H}_{2}}O$ is heated more on the platinum loop, then, a transparent colourless glass like bead of sodium metaborate \[(NaB{{O}_{3}})\] and boric anhydride \[({{\text{B}}_{2}}{{\text{O}}_{3}})\] is formed.
$N{{a}_{2}}{{B}_{4}}{{O}_{7}}.10{{H}_{2}}O\overset{\Delta }{\mathop{\to }}\,N{{a}_{2}}{{B}_{4}}{{O}_{7}}\overset{\Delta }{\mathop{\to }}\,2NaB{{O}_{2}}+{{B}_{2}}{{O}_{3}}$
So, answer is option A which is \[X=\text{N}{{\text{a}}_{2}}{{\text{B}}_{4}}{{\text{O}}_{7}}\text{, }\!\!~\!\!\text{ }Y=\text{NaB}{{\text{O}}_{2}},Z={{\text{B}}_{2}}{{\text{O}}_{3}}\]

Note: Borax, also called sodium tetraborate, is a powdery white mineral that has been used as a cleaning product for several decades. It has many uses: It helps get rid of stains, mould, and mildew around the house. It can kill insects such as ants. It is an important boron compound, a mineral, and a salt of boric acid. Powdered borax is white, consisting of soft colourless crystals that dissolve easily in water. Borax has a wide variety of uses.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




