
For the given reaction
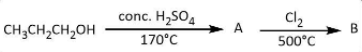
Find the products \[A\] and $B$.
A. $A:C{H_3}C{H_2}C{H_3},B:C{H_3}C{H_2}C{H_2}Cl$
B. $A:C{H_3}CH = C{H_2},B:C{H_2}ClCH = C{H_2}$
C. $A:C{H_2} = C{H_2},B:C{H_3}C{H_2}Cl$
D. $A:C{H_3}C{H_2}C{H_3},B:C{H_3}CH = C{H_2}$

Answer
574.2k+ views
Hint: We know that products can be deduced by using the formula or structure of reactant and the suitable reagent and/or conditions for every reaction would have a definite product. Alcohols show dehydration reaction with conc. ${H_2}S{O_4}$ at high temperatures.
Complete answer
We are given a reactant with formula, $C{H_3}C{H_2}C{H_2}OH$. As we can see that it contains three carbons attached in a straight chain and there is one $ - OH$ group attached to the first carbon. We know that the name or category of a given organic compound can be deduced based on the functional group present in it by following the IUPAC guidelines for the same. According to the guidelines, $ - OH$ group is also a functional group and it gives rise to the “alcohols” category of organic compounds. So, here, the given reactant’s name can be deduced as propanol.
Now, we will have a look at the chemical reactions of alcohols for the presence of $ - OH$ group renders characteristic chemical properties to them. The first reagent that is given is conc. sulfuric acid and the reaction is being carried out at $170^\circ C$. We know that alcohols undergo dehydration when treated with conc. ${H_2}S{O_4}$ at high temperatures as per the following chemical equation:
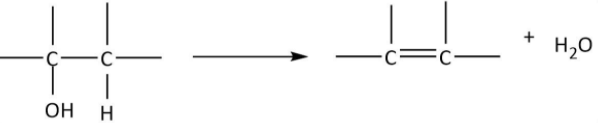
As it can be observed that hydroxyl groups along with one $H$ from the adjacent carbon get removed as water and a double bond is formed between the two carbons. We can also write the chemical equation for propanol in the similar manner and deduce the formula of product \[A\]:
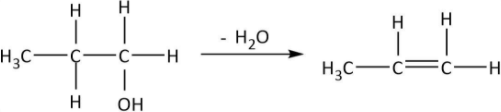
So, the product from dehydration \[\left( {A:C{H_3}CH = C{H_2}} \right)\] is an alkene. Now, it has been given that \[C{H_3}CH = C{H_2}\] is treated with chlorine at $500^\circ C$. The reaction can be shown by the following equation:
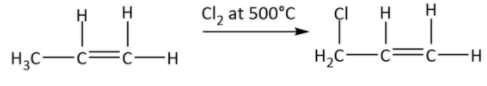
We know that reaction with chlorine at high temperature results in allylic chlorination and thus we get the product $B:C{H_2}ClCH = C{H_2}$.
Hence, the correct option is B.
Note:
We have to take into account the reaction conditions as well along with the reagents to deduce the product. The temperature and concentration of $H_2SO_4$ for obtaining is a necessary condition for the dehydration process and should not be altered.
Complete answer
We are given a reactant with formula, $C{H_3}C{H_2}C{H_2}OH$. As we can see that it contains three carbons attached in a straight chain and there is one $ - OH$ group attached to the first carbon. We know that the name or category of a given organic compound can be deduced based on the functional group present in it by following the IUPAC guidelines for the same. According to the guidelines, $ - OH$ group is also a functional group and it gives rise to the “alcohols” category of organic compounds. So, here, the given reactant’s name can be deduced as propanol.
Now, we will have a look at the chemical reactions of alcohols for the presence of $ - OH$ group renders characteristic chemical properties to them. The first reagent that is given is conc. sulfuric acid and the reaction is being carried out at $170^\circ C$. We know that alcohols undergo dehydration when treated with conc. ${H_2}S{O_4}$ at high temperatures as per the following chemical equation:

As it can be observed that hydroxyl groups along with one $H$ from the adjacent carbon get removed as water and a double bond is formed between the two carbons. We can also write the chemical equation for propanol in the similar manner and deduce the formula of product \[A\]:

So, the product from dehydration \[\left( {A:C{H_3}CH = C{H_2}} \right)\] is an alkene. Now, it has been given that \[C{H_3}CH = C{H_2}\] is treated with chlorine at $500^\circ C$. The reaction can be shown by the following equation:
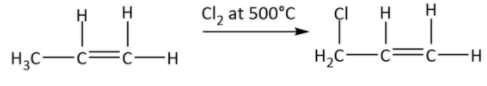
We know that reaction with chlorine at high temperature results in allylic chlorination and thus we get the product $B:C{H_2}ClCH = C{H_2}$.
Hence, the correct option is B.
Note:
We have to take into account the reaction conditions as well along with the reagents to deduce the product. The temperature and concentration of $H_2SO_4$ for obtaining is a necessary condition for the dehydration process and should not be altered.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




