
For the following reaction which of the following is/are correct?
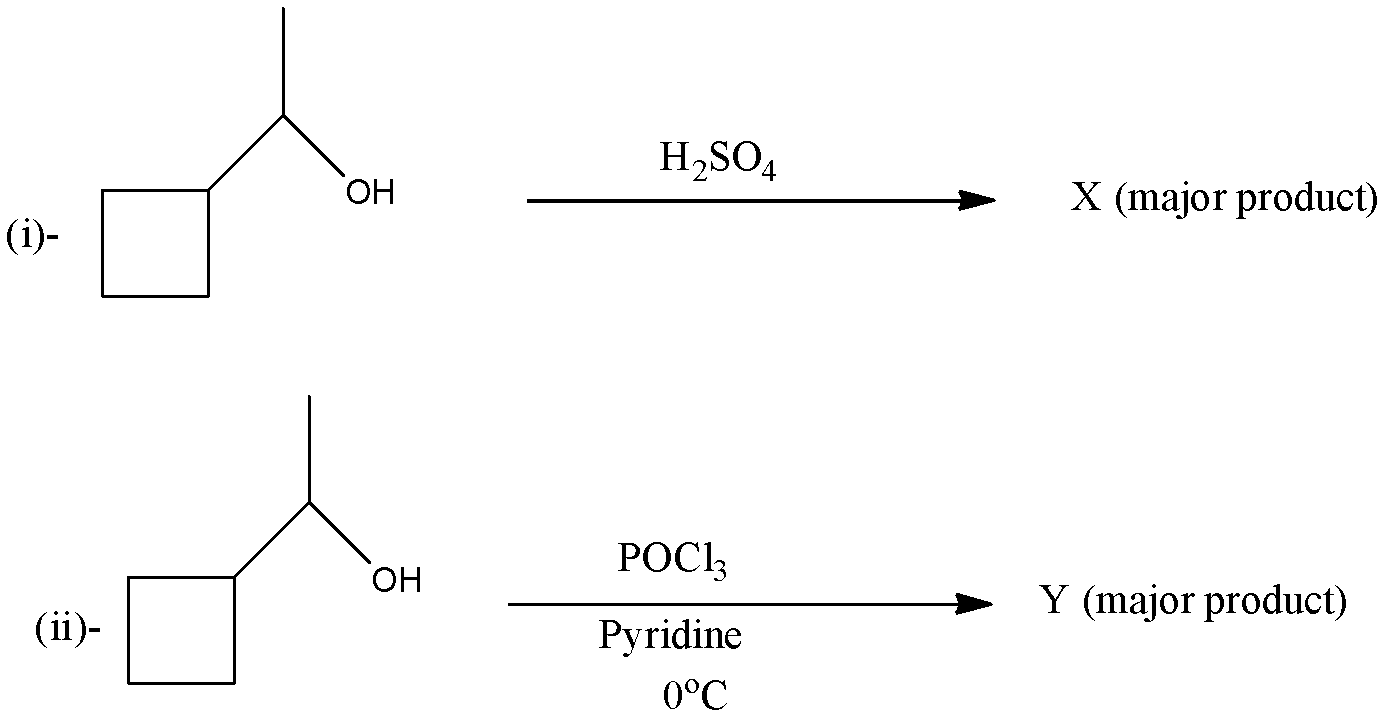
(a)- Both X and Y are five membered ring
(b)- Reaction (i) follows ${{E}_{2}}$ path whereas reaction (ii) follows ${{E}_{1}}$ path
(c)- Reaction (ii) has carbocation as an intermediate
(d)- Carbocation rearrangement takes place in reaction (i)

Answer
508.8k+ views
Hint: In both reactions, there will be a formation of alkene because there is an elimination reaction and there will be the removal of water molecules. The reaction in which carbocation is formed will be the ${{E}_{1}}$ path and the other will follow the ${{E}_{2}}$ path.
Complete answer:
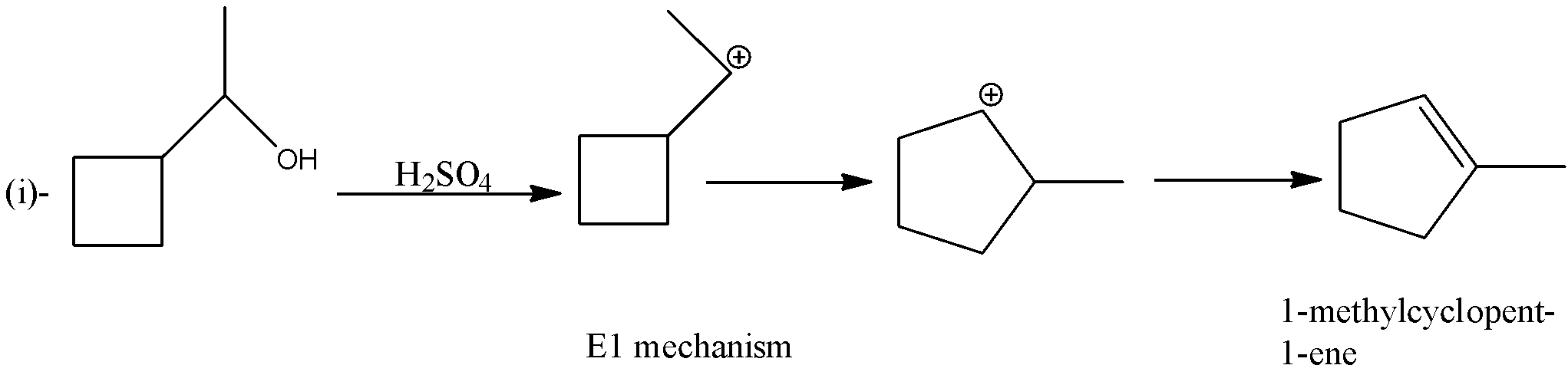
The given reactant in the reactions is 1- cyclobutylethanol.
In the first reaction, 1- cyclobutylethanol is treated with sulfuric acid, so the hydroxyl ion will be removed which will form a carbocation. There will be rearrangement of carbocation and there will be the formation of alkene. The product formed will be 1-methylcyclopent-1-ene. The reaction is given below:
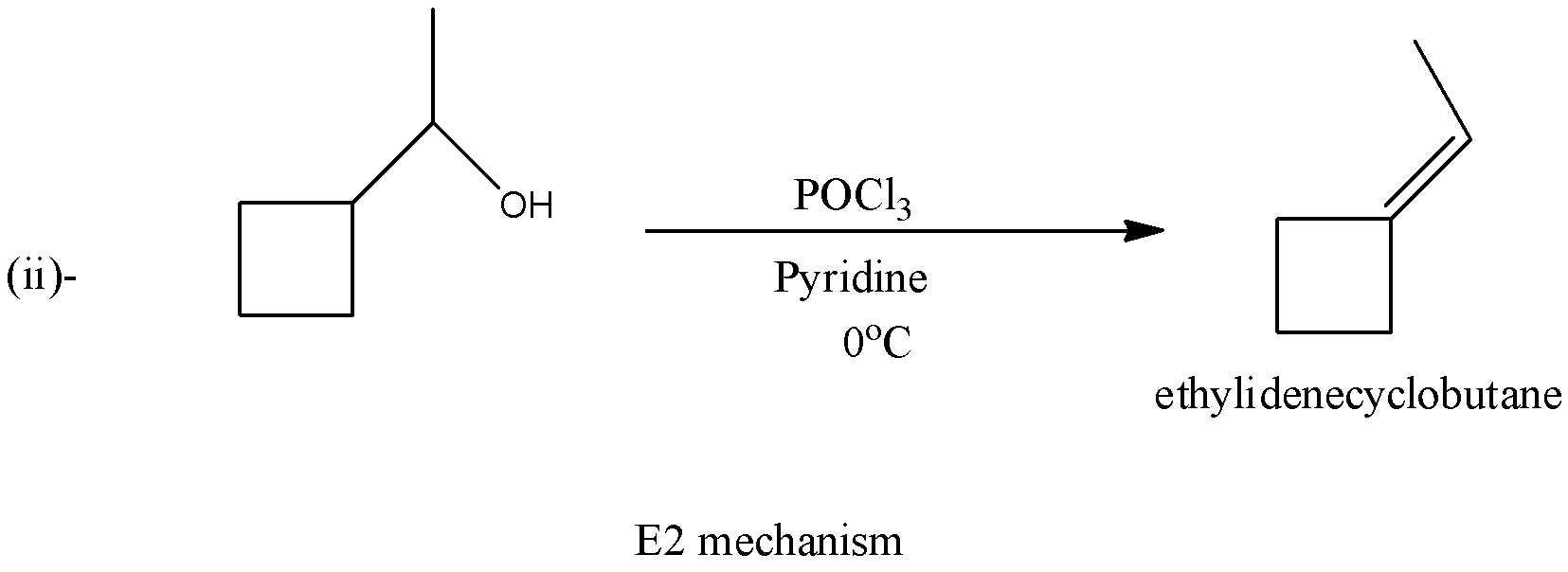
In the second reaction, there will be the formation of ethylene cyclobutane and there will be no formation of a carbocation. The reaction is given below:
So, as we can see, in the first reaction there are five membered rings and in the second reaction, there is a four-membered ring. The first reaction follows the ${{E}_{1}}$ mechanism and the second reaction follows the ${{E}_{2}}$ mechanism.
Therefore, from the given options only option (d)- Carbocation rearrangement takes place in reaction (i) is correct in the given reactions.
Hence, the correct answer is an option (d).
Note:
The rearrangement of carbocation will take place only when there is a possibility of a more stable carbocation. A tertiary carbocation is most stable while primary carbocation is the least stable.
Complete answer:
The given reactant in the reactions is 1- cyclobutylethanol.
In the first reaction, 1- cyclobutylethanol is treated with sulfuric acid, so the hydroxyl ion will be removed which will form a carbocation. There will be rearrangement of carbocation and there will be the formation of alkene. The product formed will be 1-methylcyclopent-1-ene. The reaction is given below:

In the second reaction, there will be the formation of ethylene cyclobutane and there will be no formation of a carbocation. The reaction is given below:

So, as we can see, in the first reaction there are five membered rings and in the second reaction, there is a four-membered ring. The first reaction follows the ${{E}_{1}}$ mechanism and the second reaction follows the ${{E}_{2}}$ mechanism.
Therefore, from the given options only option (d)- Carbocation rearrangement takes place in reaction (i) is correct in the given reactions.
Hence, the correct answer is an option (d).
Note:
The rearrangement of carbocation will take place only when there is a possibility of a more stable carbocation. A tertiary carbocation is most stable while primary carbocation is the least stable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




