
For the compounds the order of basicity is
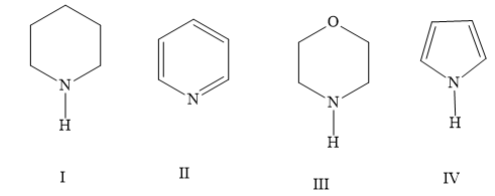
A) IV>I > III > II
B) III >I> IV > II
C) II > I > III > IV
D) I > III > II > IV

Answer
578.4k+ views
Hint:On Considering various factors such as the type of hybrid orbital ( \[s{p^3}\] or \[s{p^2}\] ) in which the lone pair of electrons of nitrogen atom is present. Also we find out if this lone pair of electrons on the nitrogen atom is involved in resonance with other pi electrons or not.
Complete answer:
In the compound I (piperidine) the lone pair of electrons on nitrogen atoms is present in an \[s{p^3}\]hybridized orbital. No resonance is present in piperidine. Hence, piperidine is the most basic among the given compounds.
In the compound II (pyridine) the lone pair of electrons on nitrogen atoms is present in an \[s{p^2}\]hybridized orbital. This lone pair of electrons is not involved in resonance as it is in a perpendicular position. Hence, piperidine is more basic than compounds III and IV.
Compared to a \[s{p^3}\] hybrid orbital, the size of an \[s{p^2}\] hybrid orbital is smaller. This leads to greater attraction between the nucleus and the lone pair of electrons on nitrogen. Hence, pyridine has less basicity than piperidine.
In the compound III (morpholine) the lone pair of electrons on nitrogen atoms is present in an \[s{p^3}\]hybridized orbital. No resonance is present in piperidine. But the oxygen atom present in the ring shows electron withdrawing effect. Due to this, the lone pair of electrons on nitrogen atoms is less available for donation. Hence, morpholine is more basic than pyridine but less basic than piperidine.
In the compound IV (pyrrole), the lone pair of electrons on the nitrogen atom is involved in the resonance with the aromatic system. When pyrrole nitrogen is protonated, the aromaticity is disturbed which leads to decrease in the stability. This makes this process unfavorable. Hence, pyrrole is least basic among the given compounds.
Hence, the option (D) I > III > II > IV is the correct answer.
Note:For basicity, the lone pair of electrons on the nitrogen atom should be easily donated to a proton. This is possible if the attraction between the nucleus and this lone pair of electrons is less. Also, it is desirable that this lone pair of electrons should not be delocalized.
Complete answer:
In the compound I (piperidine) the lone pair of electrons on nitrogen atoms is present in an \[s{p^3}\]hybridized orbital. No resonance is present in piperidine. Hence, piperidine is the most basic among the given compounds.
In the compound II (pyridine) the lone pair of electrons on nitrogen atoms is present in an \[s{p^2}\]hybridized orbital. This lone pair of electrons is not involved in resonance as it is in a perpendicular position. Hence, piperidine is more basic than compounds III and IV.
Compared to a \[s{p^3}\] hybrid orbital, the size of an \[s{p^2}\] hybrid orbital is smaller. This leads to greater attraction between the nucleus and the lone pair of electrons on nitrogen. Hence, pyridine has less basicity than piperidine.
In the compound III (morpholine) the lone pair of electrons on nitrogen atoms is present in an \[s{p^3}\]hybridized orbital. No resonance is present in piperidine. But the oxygen atom present in the ring shows electron withdrawing effect. Due to this, the lone pair of electrons on nitrogen atoms is less available for donation. Hence, morpholine is more basic than pyridine but less basic than piperidine.
In the compound IV (pyrrole), the lone pair of electrons on the nitrogen atom is involved in the resonance with the aromatic system. When pyrrole nitrogen is protonated, the aromaticity is disturbed which leads to decrease in the stability. This makes this process unfavorable. Hence, pyrrole is least basic among the given compounds.
Hence, the option (D) I > III > II > IV is the correct answer.
Note:For basicity, the lone pair of electrons on the nitrogen atom should be easily donated to a proton. This is possible if the attraction between the nucleus and this lone pair of electrons is less. Also, it is desirable that this lone pair of electrons should not be delocalized.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




