
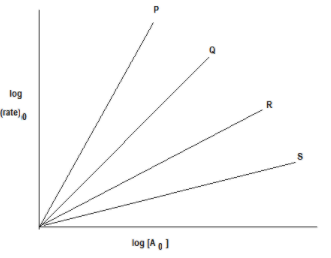
For \[{{\text{n}}^{{\text{th}}}}\] order reaction \[\left( {\dfrac{{{\text{dx}}}}{{{\text{dt}}}}} \right) = {\text{Rate = k[A}}{{\text{]}}_{\text{0}}}^{\text{n}}\] Graph between \[{\text{log (rate) }}\] against \[log{{\text{[A]}}_{\text{0}}}\] is of the type Lines P, Q, R, S are of the order:
A) P-0, Q-1,R-2,S-3
B) P-3, Q-2,R-1,S-1/2
C) P-1, Q-2,R-3,S-0
D) P-0, Q-3,R-2,S-1

Answer
578.4k+ views
Hint: Take a log of both sides of the equation. Using the linear straight line equation determines the slope of the lines. By observing the effect of change in concentration on rate of reaction determines the values of order for each line P, Q, R and S.
Complete step by step answer:
For \[{{\text{n}}^{{\text{th}}}}\] order reaction rate equation given to us is:
\[\left( {\dfrac{{{\text{dx}}}}{{{\text{dt}}}}} \right) = {\text{Rate = k[A}}{{\text{]}}_{\text{0}}}^{\text{n}}\]
So, \[{\text{Rate = k[A}}{{\text{]}}_{\text{0}}}^{\text{n}}\]
Here,
n = order of reaction
k = rate constant
Now, take a log of both sides of the equation.
\[{\text{log(Rate ) = n log [A}}{{\text{]}}_{\text{0}}} + \operatorname{logk} \]
Also, we have given a graph between \[{\text{log (rate) }}\]against \[log{{\text{[A]}}_{\text{0}}}\] as follows:
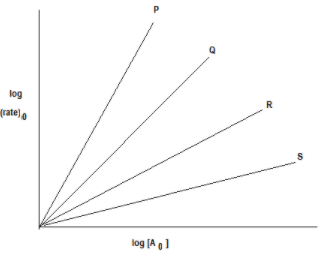
From the plot \[{\text{log (rate) }}\]against, \[log{{\text{[A]}}_{\text{0}}}\]we can say that all lines P, Q, R and S follow straight-line equations.
Mathematical straight line equation is:
\[{\text{y = mx + c }}\]
Now, comparing the straight-line equation with \[{\text{log(Rate ) = n log [A}}{{\text{]}}_{\text{0}}} + \operatorname{logk} \] equation we can say that
\[{\text{y =log(Rate )}}\]
\[{\text{x= log [A}}{{\text{]}}_{\text{0}}}\]
\[{\text{m = slope = n}}\]
\[{\text{c }} = {\text{ intercept =}}\operatorname{logk} \]
Now, by observing the slope of lines we can determine the order of lines P, Q, R and S as follows:
The order of reaction is defined as the number of molecules whose concentration alters as a result of chemical change.
A reaction is said to be zero-order if the rate of reaction is independent of the concentration of the reactants. None of the lines in the graph indicates that there is no effect of change in concentration on the rate of reaction. So none of the lines are zero order.
So, options A, C and D are incorrect.
A reaction whose rate is determined by the change of one concentration term only is known as the first-order reaction. In the case of first-order reaction rate of reaction is directly proportional to the change in concentration. From the graph, we can say the order of line R is 1 as a change in concentration and change in the rate of reaction is directly proportional, that is doubling the concentration of reactant doubles the rate of reaction.
Order of line S is 1/2 as here the rate of reaction is half to that of concentration A. We can say that as the slope of the line S is 1/2 so the order of line is 1/2.
Order of line Q is 2 as the rate of reaction doubles to that of concentration A. We can say that as the slope of line Q is 2 so the order of line is 2.
Order of line P is 3 as here the rate of reaction thrice to that of concentration A. We can say that as the slope of line P is 3 so the order of line is 3.
So, the order of reaction with respect to P, Q, R and S is 3, 2, 1 and 1/2 respectively.
Thus, the correct option is (B) P-3, Q-2, R-1, S- 1/2
Note: The rate of reaction is the change in concentration of reactant or product in unit time. Change in concentration of reactants affects the rate of reaction. The mathematical relation between the rate of reaction and the concentration of the reaction component is known as the rate law expression. To determine the rate law of reaction it is necessary to calculate the order of the reaction.
Complete step by step answer:
For \[{{\text{n}}^{{\text{th}}}}\] order reaction rate equation given to us is:
\[\left( {\dfrac{{{\text{dx}}}}{{{\text{dt}}}}} \right) = {\text{Rate = k[A}}{{\text{]}}_{\text{0}}}^{\text{n}}\]
So, \[{\text{Rate = k[A}}{{\text{]}}_{\text{0}}}^{\text{n}}\]
Here,
n = order of reaction
k = rate constant
Now, take a log of both sides of the equation.
\[{\text{log(Rate ) = n log [A}}{{\text{]}}_{\text{0}}} + \operatorname{logk} \]
Also, we have given a graph between \[{\text{log (rate) }}\]against \[log{{\text{[A]}}_{\text{0}}}\] as follows:

From the plot \[{\text{log (rate) }}\]against, \[log{{\text{[A]}}_{\text{0}}}\]we can say that all lines P, Q, R and S follow straight-line equations.
Mathematical straight line equation is:
\[{\text{y = mx + c }}\]
Now, comparing the straight-line equation with \[{\text{log(Rate ) = n log [A}}{{\text{]}}_{\text{0}}} + \operatorname{logk} \] equation we can say that
\[{\text{y =log(Rate )}}\]
\[{\text{x= log [A}}{{\text{]}}_{\text{0}}}\]
\[{\text{m = slope = n}}\]
\[{\text{c }} = {\text{ intercept =}}\operatorname{logk} \]
Now, by observing the slope of lines we can determine the order of lines P, Q, R and S as follows:
The order of reaction is defined as the number of molecules whose concentration alters as a result of chemical change.
A reaction is said to be zero-order if the rate of reaction is independent of the concentration of the reactants. None of the lines in the graph indicates that there is no effect of change in concentration on the rate of reaction. So none of the lines are zero order.
So, options A, C and D are incorrect.
A reaction whose rate is determined by the change of one concentration term only is known as the first-order reaction. In the case of first-order reaction rate of reaction is directly proportional to the change in concentration. From the graph, we can say the order of line R is 1 as a change in concentration and change in the rate of reaction is directly proportional, that is doubling the concentration of reactant doubles the rate of reaction.
Order of line S is 1/2 as here the rate of reaction is half to that of concentration A. We can say that as the slope of the line S is 1/2 so the order of line is 1/2.
Order of line Q is 2 as the rate of reaction doubles to that of concentration A. We can say that as the slope of line Q is 2 so the order of line is 2.
Order of line P is 3 as here the rate of reaction thrice to that of concentration A. We can say that as the slope of line P is 3 so the order of line is 3.
So, the order of reaction with respect to P, Q, R and S is 3, 2, 1 and 1/2 respectively.
Thus, the correct option is (B) P-3, Q-2, R-1, S- 1/2
Note: The rate of reaction is the change in concentration of reactant or product in unit time. Change in concentration of reactants affects the rate of reaction. The mathematical relation between the rate of reaction and the concentration of the reaction component is known as the rate law expression. To determine the rate law of reaction it is necessary to calculate the order of the reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




