
For given complex [\[Ni{\left( {PP{h_3}} \right)_{2}}C{l_2}\]] mention
(i) IUPAC Name
(ii) Central metal ion
(iii) Ligands
(iv) Coordination number
(v) Nature of the complex
Answer
579.6k+ views
Hint: [\[Ni{\left( {PP{h_3}} \right)_{2}}C{l_2}\]], dark blue coloured crystalline solid, is a metal phosphine complex which is generally employed as a catalyst for organic synthesis.
Complete step by step answer:
The structure of the given complex is shown below:
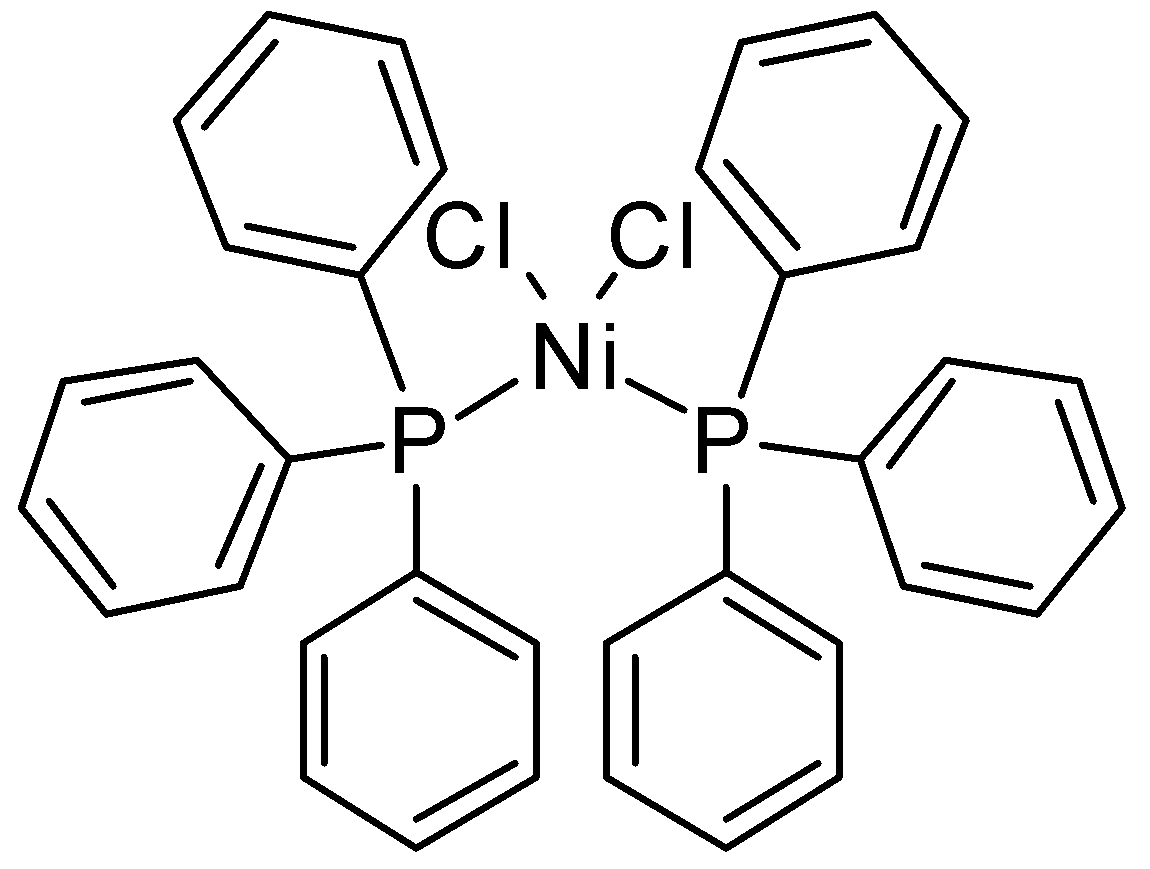
(i) IUPAC Name: Dichlorobis(triphenylphosphine)nickel(II) is the IUPAC name for [\[Ni{\left( {PP{h_3}} \right)_{2}}C{l_2}\]]
(ii) Central metal ion: Central metal ion is the metal ion with which ligands are being attached at the centre of the coordination complex. It is clear from the structure that the central metal ion is \[N{i^{2 + }}\].
(iii) Ligands: A ligand refers to either an ion or a molecule (or simply functional group) which binds to a central metal ion or atom in order to create a coordination complex. It is clearly visible in the structure of the given complex that the ligands are \[PP{h_3}\]and \[C{l^ - }\].
(iv) Coordination number: Coordination number refers to the number of ions, atoms or molecules which a central metal ion or atom holds as its nearest neighbours in a coordination complex. In the present case, coordination number is 4.
(v) Nature of the complex: As no charge is present in the given complex, nature of the complex is neutral.
Hint:
Always keep in mind that the formula for a coordination complex has to be written in a different manner as compared to its name. Firstly, write the chemical symbol of the central metal atom or ion. Then, write the ligands name with the anion ligands coming before the neutral ligands.
Complete step by step answer:
The structure of the given complex is shown below:

(i) IUPAC Name: Dichlorobis(triphenylphosphine)nickel(II) is the IUPAC name for [\[Ni{\left( {PP{h_3}} \right)_{2}}C{l_2}\]]
(ii) Central metal ion: Central metal ion is the metal ion with which ligands are being attached at the centre of the coordination complex. It is clear from the structure that the central metal ion is \[N{i^{2 + }}\].
(iii) Ligands: A ligand refers to either an ion or a molecule (or simply functional group) which binds to a central metal ion or atom in order to create a coordination complex. It is clearly visible in the structure of the given complex that the ligands are \[PP{h_3}\]and \[C{l^ - }\].
(iv) Coordination number: Coordination number refers to the number of ions, atoms or molecules which a central metal ion or atom holds as its nearest neighbours in a coordination complex. In the present case, coordination number is 4.
(v) Nature of the complex: As no charge is present in the given complex, nature of the complex is neutral.
Hint:
Always keep in mind that the formula for a coordination complex has to be written in a different manner as compared to its name. Firstly, write the chemical symbol of the central metal atom or ion. Then, write the ligands name with the anion ligands coming before the neutral ligands.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




